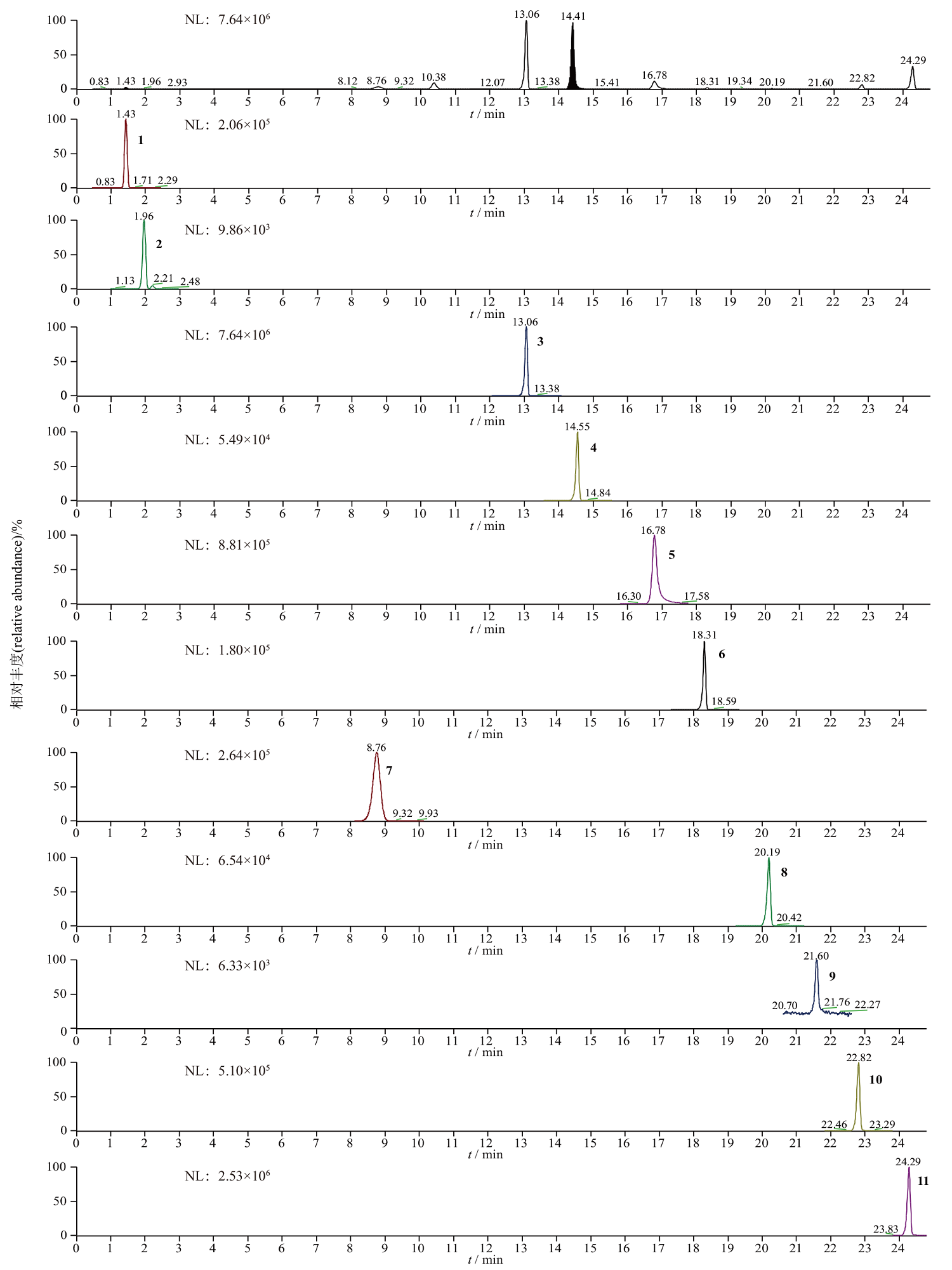
Objective: To establish an HPLC-MS/MS method for simultaneous determination of 11 components (harpagide, salidroside, nuezhenide, lobetyolin, wedelolactone, harpagoside, vaccarin, 6-gingerol, atractylenolide Ⅲ, atractylenolide Ⅱ, and atractylenolide Ⅰ) in Gengnianning. Methods: HPLC assay was performed on C18 column(100 mm×2.1 mm, 1.9 μm) with a mixture of methanol and 0.1% formic acid as the mobile phase in gradient elution at a flow rate of 0.3 mL·min-1.The column temperature was 25 ℃ and the injection volume was 1 μL. Detection was carried out on a triple quadrupole mass spectrometer in positive ion mode (harpagide, salidroside, nuezhenide, lobetyolin, wedelolactone and harpagoside) and negative ion mode(vaccarin, 6-gingerol, atractylenolide Ⅲ, atractylenolide Ⅱ, and atractylenolide Ⅰ) using an electrospray ion source(ESI). Multiple reaction monitoring(MRM)mode was employed. Results: The calibration curves were linear within the ranges of 1.485-29.71 μg·mL-1, 1.620-32.40 μg·mL-1, 7.801-156.0 μg·mL-1, 0.518-10.35 μg·mL-1, 0.167-3.333 μg·mL-1, 0.359-7.179 μg·mL-1, 1.455-29.10 μg·mL-1, 1.520-30.40 μg·mL-1, 0.160-3.205 μg·mL-1, 0.143-2.864 μg·mL-1 and 0.157-3.136 μg·mL-1 for harpagide, salidroside, nuezhenide, lobetyolin, wedelolactone, harpagoside, vaccarin, 6-gingerol, atractylenolide Ⅲ, atractylenolide Ⅱ, and atractylenolide Ⅰ, respectively. All 11 components showed good linearity (r≥0.998 0). The average recoveries(n=6)were in the range of 95.9%-102.6% with RSDs within 0.90%-3.0%. The contents of harpagide, salidroside, nuezhenide, lobetyolin, wedelolactone, harpagoside, vaccarin, 6-gingerol, atractylenolide Ⅲ, atractylenolide Ⅱ and atractylenolide Ⅰ in 10 tested samples from 5 manufactures were 14.8-104.5, 37.6-288.5, 335.8-1 332.8, 6.2-10.1, 6.6-61.8, 13.7-75.1, 57.4-132.8, 16.9-70.6, 11.8-33.9, 3.4-15.4 and 6.5-12.9 μg·g-1. Conclusion: The developed method is accurate and sensitive. It can be used in quality control of Gengnianning.
[1] 卫生部药品标准中药成方制剂第6册 [S]. 1993:70
Drug Specifications Promulgated by Ministry of Public Health. Prescription of Chinese Patent Medicine Vol 6[S]. 1993:70
[2] 郑阿旭,鲁晓光.HPLC-PDA法同时测定更年宁中芍药苷、阿魏酸、黄芩苷和丹皮酚的含量[J]. 中南药学,2020,18(2):258
ZHENG AX, LU XG. Simultaneous determination of paeoniflorin, ferulic acid, baicalin and paeonol in Gengnianning pills by HPLC-PDA[J]. Cent South Pharm, 2020, 18(2):258
[3] 石洪超,杨能英,何风雷,等.更年宁质量控制方法的建立研究[J]. 中国当代医药,2017,24(20):141
SHI HC, YANG NY, HE FL, et al. Study on establishment of quality control method for Gengnianning [J]. China Mod Med, 2017, 24(20):141
[4] 潘炎铃.RP-HPLC测定更年宁中黄芩苷的含量[J]. 人参研究,2011,23(3):24
PAN YL. Determination of baicalin in Genanning by RP-HPLC[J]. Ginseng Res, 2011, 23(3):24
[5] 张勇纯,刘彦玲,王铭爽,等.基于内在成分含量差异辨别玄参药材品质优劣可行性研究[J]. 中草药,2022,53(2):544
ZHANG YC, LIU YL, WANG MS, et al. Feasibility study on quality of Scrophulariae Radix based on chemical component quantitative difference analysis[J]. Chin Tradit Herb Drugs, 2022, 53(2):544
[6] 任丹,齐方圆,黄紫妍,等.15个产地玄参中哈巴苷与哈巴俄苷含量测定[J]. 药学实践杂志,2021,39(4):313
REN D, QI FY, HUANG ZY, et al. Determination of harpagide and harpagoside contents in Scrophulariae Radix from 15 origins[J]. J Pharm Pract, 2021, 39(4):313
[7] 张柏生,李斌,李茜,等.不同产地酒女贞子红景天苷、特女贞苷含量测定及聚类分析[J]. 世界中医药,2021,16(3):426
ZHANG BS, LI B, LI Q, et al. Determination of salidroside and nuezhenide in different producing areas and cluster analysis[J]. World Chin Med, 2021, 16(3):426
[8] 纪鑫,刘晓谦,李春,等.基于UPLC技术的女贞子中主要成分炮制变化规律研究及酒女贞子特色质量标准的探讨[J]. 中国中药杂志,2018,43(24):4862
JI X, LIU XQ, LI C, et al. Content change of main components in processed Ligustrum lucidum fruits of different processing time and their characteristic quality standard based on UPLC technology[J]. China J Chin Mater Med, 2018, 43(24):4862
[9] 张小婷,张芮铭,牛媛婧,等.基于多成分含量测定及化学计量学的党参质量评价[J]. 中药材,2022,45(10):2411
ZHANG XT, ZHANG RM, NIU YJ, et al. Quality evaluation of Codonopsis Radix based on multi-component content determination and chemometrics[J]. J Chin Med Mater, 2022, 45(10):2411
[10] 刘颖新,冯传平,刘利利,等.一测多评法同时测定不同产地墨旱莲中9种成分及其质量评价研究[J]. 中国现代应用药学,2022,39(18):2339
LIU YX, FENG CP, LIU LL, et al. Study on simultaneous determination of 9 components in Ecliptae Herba from different growing areas by QAMS method and it’s quality evaluation[J]. Chin J Mod Appl Pharm, 2022, 39(18):2339
[11] 黄懿,欧阳波,肖作奇,等.不同产地炒王不留行HPLC指纹图谱及含量测定研究[J]. 中南药学,2017,15(7):879
HUANG Y, OUYANG B, XIAO ZQ, et al. Content determination and fingerprint of fried Vaccaria segetalis from different habitats by HPLC[J]. Cent South Pharm, 2017, 15(7):879
[12] 何蕊,刘鹤,翟俊民,等.不同产地干姜中6-姜辣素含量的考察[J]. 中国地方病防治杂志,2018,33(6):647
HE R, LIU H, ZHAI JM, et al. Study the content of 6-gingerol in Zingiberis Rhizoma from different places [J]. Chin J Control Endem Dis, 2018, 33(6):647
[13] 杜晓蕾,崔伟亮,朱日然,等.指纹图谱结合化学计量学研究白术麸炒前后成分变化情况[J]. 中药材,2022,45(10):2358
DU XL, CUI WL, ZHU RR, et al. Study the changes of Atractylodis Macrocephalae Rhizoma ingredients before and after stir-frying by chromatographic fingerprint combined with chemometrics [J]. J Chin Med Mater, 2022, 45(10):2358
[14] 高红宁,潘雨柔,殷奕,等.高效液相色谱法测定不同产地麸炒白术中苍术酮、白术内酯Ⅰ、白术内酯Ⅱ和白术内酯Ⅲ的含量[J]. 中国当代医药,2021,28(3):4
GAO HN, PAN YR, DUAN Y, et al. Content determination of atractylone, atractylenolide Ⅰ, atractylenolide Ⅱ and atractylenolide Ⅲ in Atractylodis Macrocephalae Rhizoma stir-fried with wheat bran from different habitats by high performance liquid chromatogram method[J]. China Mod Med, 2021, 28(3):4
[15] 林伟雄,邓李红,李美洲,等.基于UPLC特征图谱及多成分同时定量的酒女贞子炮制工艺研究[J]. 南京中医药大学学报,2022,38(3):236
LIN WX, DENG LH, LI MZ, et al. Study on processing procedure of wine-processed Ligustri Lucidi Fructus based on UPLC characteristic chromatogram and multicomponent content determination[J]. J Nanjing Univ Tradit Chin Med, 2022, 38(3):236
[16] 纪鑫. 女贞子产地加工、炮制中化学成分变化规律及酒女贞子特色质量标准探索研究[D]. 北京:中国中医科学院,2019
JI X. Studies on The Transformation of Chemical Components in The Processing of Ligustri Lucidi Fructus and Characteristic Quality Standard of Wine-steamed Ligustri Lucidi Fructus[D]. Beijing:China Academy of Chinese Medical Sciences, 2019
[17] 纪鑫,刘晓谦,李春,等.基于UPLC技术的女贞子中主要成分炮制变化规律研究及酒女贞子特色质量标准的探讨[J]. 中国中药杂志,2018,43(24):4862
JI X, LIU XQ, LI C, et al. Content change of main components in processed Ligustrum lucidum fruits of different processing time and their characteristic quality standard based on UPLC technology[J]. China J Chin Mater Med, 2018, 43(24):4862


