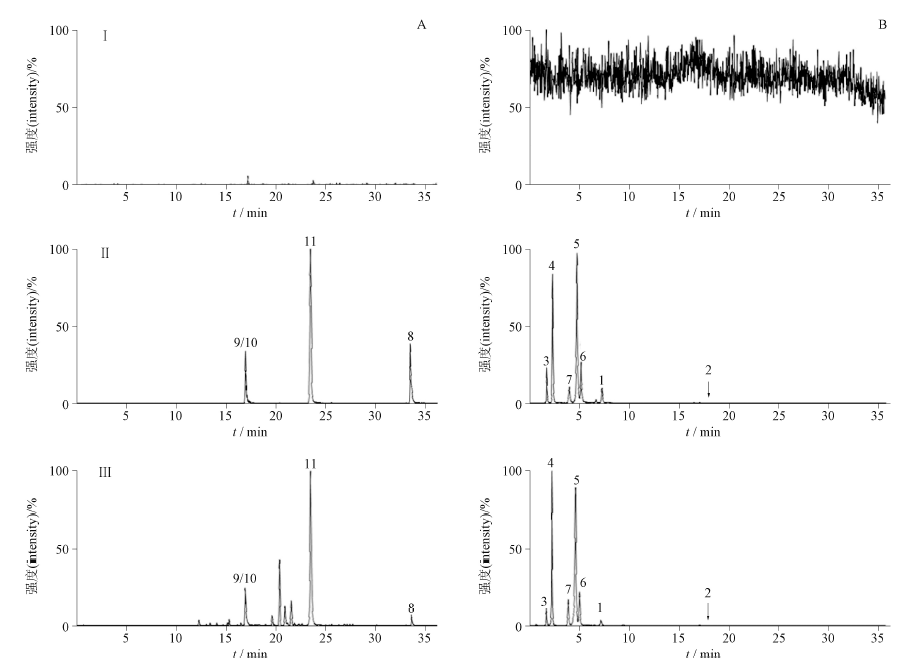
Objective: To establish an UPLC-MS/MS method for simultaneous determination of 11 components(caffeic acid, afzelin, gallic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, salidroside, eleutheroside B, ginsenoside Re, ginsenoside Rg1, ginsenoside Rd) in Hongwushen capsules. Methods: A Shim-pack GIST C18 chromatographic column(100 mm×2.1 mm, 2 μm) was used. The mobile phase was composed of acetonitrile-0.1% acetic acid-1 mmol·L-1 ammonium acetate aqueous solution and the gradient elution was applied. The flow rate was 0.3 mL·min-1, the column temperature was 30 ℃, and the injection volume was 1 μL. Electrospray ionization (ESI) source and multiple reaction monitoring (MRM) mode in both positive and negative were used for ion detections. Results: The linear relationship of 11 components was good in the concentration range, and the linear correlation coefficients were all above 0.999 0. The RSD values of precision were less than 3%. The repeatability and stability were good, and the RSD values were less than 5%. The average recoveries were in the range of 97.1%-101.5% with RSDs≤3.7%. The contents of caffeic acid, afzelin, gallic acid, neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, salidroside, eleutheroside B, ginsenoside Re, ginsenoside Rg1 and ginsenoside Rd in 10 batches of Hongwushen capsules were 0.010-0.013 mg·g-1, 0.000 7-0.001 4 mg·g-1, 0.035-0.038 mg·g-1, 0.312-0.315 mg·g-1, 0.413-0.417 mg·g-1, 0.411-0.416 mg·g-1, 4.355-4.358 mg·g-1, 0.030-0.032 mg·g-1, 0.993-0.999 mg·g-1, 1.120-1.124 mg·g-1 and 2.536-2.538 mg·g-1, respectively. Conclusions: The method is accurate, sensitive, stable and reproducible, and can be used for the quality control of Hongwushen capsules.
SUN Shuai, WANG Xiang, GAO Le, XIE Wen-chao, LIU Chen-nan, NIU Li-ying, WANG Xin-guo
. Simultaneous determination of 11 components in Hongwushen capsules by UPLC-MS/MS*[J]. Chinese Journal of Pharmaceutical Analysis, 2024
, 44(4)
: 567
-576
.
DOI: 10.16155/j.0254-1793.2024.04.03
[1] 程甲波. 刺五加抗疲劳谱效关系及质量评价研究[D]. 哈尔滨:东北林业大学, 2022
CHENG JB. Study on Anti-fatigue Spectrum Effect Relationship and Quality Evaluation of Acanthopanax senticosus[D]. Harbin: Northeast Forestry University, 2022
[2] 谢靖宇, 郭传燕. 刺五加提取物对强迫游泳小鼠的抗疲劳能力的影响[J]. 基因组学与应用生物学, 2020, 39(9):4277
XIE JY, GUO CY. Effect of Acanthopanax senticosus extract on anti-fatigue ability of forced swimming mice[J]. Genomics Appl Biol, 2020, 39(9):4277
[3] 师艺玮, 王洪玲, 黄慧莲, 等. UPLC-MS/MS法同时测定经典方剂槐花散中7个成分含量[J]. 药物分析杂志, 2023, 43(2):219
SHI YW, WANG HL, HUANG HL, et al. Simultaneous determination of 7 components in classic formula Huaihua powder by UPLC-MS/MS[J]. Chin J Pharm Anal, 2023, 43(2):219
[4] 黄如玉, 唐瑞欣, 厉言, 等. UPLC-MS/MS法同时测定连花清瘟胶囊中11种成分[J]. 中成药, 2023, 45(1):24
HUANG RY, TANG RX, LI Y, et al. Simultaneous determination of eleven constituents in Lianhua Qingwen capsules by UPLC-MS/MS[J]. Chin Tradit Pat Med, 2023, 45(1):24
[5] 郑燕芳, 林逸凡, 朱铭星, 等. UPLC-MS/MS同时测定八宝丹中15个胆酸类成分的含量[J]. 药物分析杂志, 2022, 42(12):2186
ZHENG YF, LIN YF, ZHU MX, et al. Simultaneous determination of 15 bile acids in Babaodan by UPLC-MS/MS[J]. Chin J Pharm Anal, 2022, 42(12):2186
[6] 王博, 杨忠杰, 裴媛, 等. LC-MS/MS同时测定芪桂苓合剂中7个成分[J]. 药物分析杂志, 2022, 42(12):2202
WANG B, YANG ZJ, PEI Y, et al. Simultaneous determination of seven components in Qiguiling mixture by LC-MS/MS[J]. Chin J Pharm Anal, 2022, 42(12):2202
[7] 秦宁宁, 谢华, 赵安鹏, 等. 高原低氧环境下红景天苷对小鼠运动耐力的影响[J]. 浙江大学学报(医学版), 2022,51(4):397
QIN NN, XIE H, ZHAO AP, et al. Effects of salidroside on exercise tolerance of mice under high altitude hypoxia environment[J]. J Zhejiang Univ(Med Sci), 2022, 51(4):397
[8] 刘昕. 栀子花黄酮纯化工艺及其抗氧化与抗运动疲劳作用研究[J]. 中国食品添加剂, 2023, 34(2):27
LIU X. Optimization of purification process of flavonoids of Gardenia flower and its anti-oxidant and anti-fatigue effects[J]. China Food Addit, 2023, 34(2):27
[9] XIA F, ZHONG Y, LI M, et al. Antioxidant and anti-fatigue constituents of okra[J]. Nutrients, 2015, 7(10):8846
[10] ANDRADE AWL, MACHADO KDC, MACHADO KDC, et al. In vitro antioxidant properties of the biflavonoid agathisflavone[J]. Chem Cent J, 2018, 12(1):75
[11] CHENG J, QIU L, AHMAD N, et al. Screening of anti-fatigue active ingredients of Eleutherococcus senticosus via spectrum-effect relationship based on factor analysis and LC-MS/MS[J]. Nat Prod Res, 2023, 37(24):1
[12] 彭烁. 罗非鱼抗氧化肽的制备及其抗疲劳功效研究[D]. 湛江: 广东海洋大学, 2022
PENG S. Preparation of Antioxidant Peptides from Tilapia and Its Anti-fatigue Effects Abstract[D]. Zhanjiang: Ocean University of Guangdong, 2022
[13] HUANG LZ, HUANG BK, YE Q, et al. Bioactivity-guided fractionation for anti-fatigue property of Acanthopanax senticosus[J]. J Ethnopharmacol, 2011, 133(1):213
[14] 孔凡秀. 人参皂苷Rg1通过PGC-1α途径抗疲劳及机制研究[D]. 大庆: 黑龙江八一农垦大学, 2021
KONG FX. Research on Anti-fatigue and Mechanism of Ginsenoside Rg1 through PGC-1α Pathway[D]. Daqing: Heilongjiang Bayi Agricultural University, 2021
[15] 赵丽, 吴建忠, 岳明, 等. 一次力竭运动小鼠中枢单胺类神经递质的代谢特点[J]. 中国医学创新, 2014,11(6):8
ZHAO L, WU JZ, YUE M, et al. Branin monoamines neurotransmitters metabolism induced by a prolonged exhaustive exercise in mice[J]. Med Innov China, 2014, 11(6):8
[16] 张祥, 张晶莹, 宋昕恬, 等. 人参皂苷的抗疲劳作用研究[J]. 安徽农业科学, 2018, 46(5):12
ZHANG X, ZHANG JY, SONG XT, et al. Study on anti-fatigue effects of ginsenoside[J]. J Anhui Agric Sci, 2018, 46(5):12
[17] 王丽娜, 姜珊, 王溪竹, 等. 人参茎叶中原二醇型、原三醇型人参皂苷抗疲劳作用试验[J]. 中国兽医杂志, 2019, 55(8):81
WANG LN, JIANG S, WANG XZ, et al. Study on anti-fatigue effect of protopanaxadiol-type Ginseng and protopanaxatriol-type Ginseng stem and leave saponin[J]. J Univ Vet Med, 2019, 55(8):81


