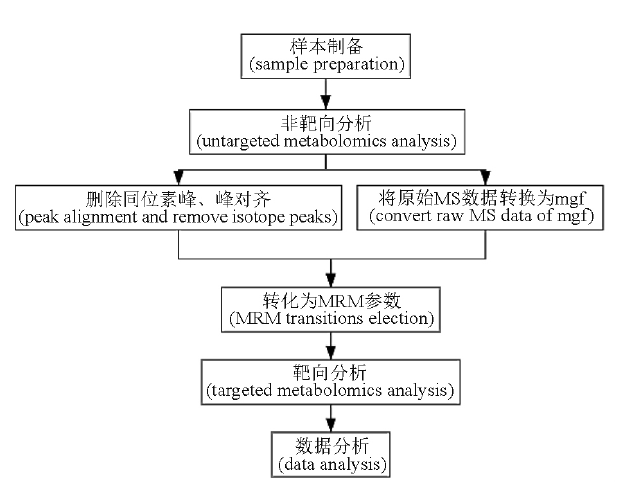
Objective: To perform a comprehensive analysis of extractables in rubber by pseudotargeted metabolomics. Methods: The quality control(QC) solutions and test solutions were prepared by extracting the six kinds of rubbers with water, pH 2.6 phosphate buffer, pH 9.18 phosphate buffer, and 15% ethanol solution, respectively. The QC solutions were detected by LC-30A ultra performance liquid chromatography-Q TOF-9030 mass spectrometer in ESI+/ESI- mode. The mobile phases were 0.1% formic acid-water and 0.1% formic acid-acetonitrile/0.05% ammonium-water and 0.05% ammonium-5% water-methanol. The separation chromatographic column was Waters Acquity BEH C8/HSS T3. TOF MS and MS/MS DDA scanning modes were used to detect the QC solutions. The scan results of QC solution were processed with MSConvert and MRM-Ion_Pair_Finder software to obtain MRM parameters. The extracts were detected by ExionLC UPLC-Qtrap6500+ mass spectrometer in ESI+/ESI- mode with the same mobile phase and column as before, and Scheduled-MRM scan mode was applied to detect the extracts. Results: The 1 399 unknown compounds and 116 known compounds were analyzed. By extraction solution, it was found that pH 9.18 phosphate buffer and 15% ethanol solution could extract more compounds, and 27 compounds with large differences in different extraction solutions were identified. By rubbers, the affinities of different rubbers were determined by 25 differences. Conclusion: In this study, the results of the extraction of six rubbers under four extracts are analyzed. There are differences between extracts under different extraction conditions. Analytical methods can be specifically developed for these key variables for quality control follow-up studies to ensure the stability and consistency of drug quality. The variables in different rubbers are also analyzed, which can be used to make a general class determination of unknown rubber samples.
SHEN Hao-wen, XIAO Meng-qing, MENG Hai-tao, LENG Xiang-yang, YAN Fang
. Analysis of extractables in rubber with pseudotargeted metabolomics method*[J]. Chinese Journal of Pharmaceutical Analysis, 2024
, 44(4)
: 637
-648
.
DOI: 10.16155/j.0254-1793.2024.04.11
[1] 李婷婷. 药用丁基胶塞与头孢唑林钠相容性研究[D]. 南昌: 南昌大学, 2011
LI TT. Study on the Compatibility between Butyl Rubber Closuer and Cefazolin Sodium[D]. Nanchang: Nanchang University, 2011
[2] 赵霞. 药用丁基胶塞与头孢曲松钠的相容性研究[D]. 北京: 中国协和医科大学, 2006
ZHAO X. Study on the Compatibility between Butyl Rubber and Ceftriaxone Sodium[D]. Beijing: Peking Union Medical College, 2006
[3] GUO P, LI X, WANG J, et al. Study on the compatibility of cefotaxime with tinidazole in glucose injection[J] J Pharm Biomed Anal, 2007, 43(5):1849
[4] 直接接触药品的包装材料和容器管理办法[S]. 2005: 44
Direct Contact Drug Packaging Material and Container Management Measures[S]. 2005: 44
[5] 化学药品注射剂与塑料包装材料相容性研究技术指导原则[S]. 2012
Technical Guidance Principles for the Study of the Compatibility of Chemical Drug Injection with Plastic Packaging Materials[S]. 2012
[6] 化学药品注射剂与药用玻璃包装容器相容性研究技术指导原则(试行)[S]. 2015
Technical Guidance Principles for the Study of the Compatibility of Chemical Drug Injection with Glass Packaging Containers (Trial Implementation)[S]. 2015
[7] 化学药品与弹性体密封件相容性研究技术指导原则(试行)[S]. 2018
Technical Guidance Principles for the Study of the Compatibility of Chemical Drugs and Elastic Seal Components (Trial Implementation)[S]. 2018
[8] 张芳芳, 杨美成, 蔡荣, 等. 《美国药典》药包材标准体系概况与最新进展[J]. 医药导报, 2023, 42(7):1009
ZHANG FF, YANG MC, CAI R, et al. Overview and latest progress of pharmaceutical packaging standard system in the United States Pharmacopoeia[J]. Her Med, 2023, 42(7):1009
[9] ISHIKAWA K. Performance and safety requirements for medical rubber components[J]. Int Polymer Sci Technol, 2015, 42(6):T37
[10] NICHOLSON JK, LINDON JC. Systems biology: metabonomics[J]. Nature, 2008, 455(7216):1054
[11] CAJKA T. FIEHN O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics[J]. Anal Chem, 2016, 88(1):524
[12] LI Y, RUAN Q, LI Y, et al. A novel approach to transforming a non-targeted metabolic profiling method to a pseudo-targeted method using the retention time locking gas chromatography/mass spectrometry-selected ions monitoring[J]. J Chromatogr A, 2012, 1255(14):228
[13] CHEN S, KONG H, LU X, et al. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry[J]. Anal Chem, 2013, 85(17):8326
[14] LI G, SEYMOUR AB, WEI R. High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics[J]. Anal Chem, 2010, 82(13):5527
[15] LU W, BENNETT BD, RABINOWITZ JD. Analytical strategies for LC-MS-based targeted metabolomics[J]. J Chromatogr B Anal Technol Biomed Life Sci, 2008, 871(2):236
[16] TSUGAWA H, TSUJIMOTO Y, SUGITATE K, et al. Highly sensitive and selective analysis of widely targeted metabolomics using gas chromatography/triple-quadrupole mass spectrometry[J]. J Biosci Bioeng, 2014, 117(1):122
[17] ZHENG F, ZHAO X, ZENG Z, et al. Development of a plasma pseudotargeted metabolomics method based on ultra-high-performance liquid chromatography-mass spectrometry[J]. Nat Protoc, 2020, 15(8):1
[18] LUO P, DAI W, YIN P, et al. Multiple reaction monitoring-ion pair finder: a systematic approach to transform nontargeted mode to pseudotargeted mode for metabolomics study based on liquid chromatography-mass spectrometry[J]. Anal Chem, 2015, 87(10):5050
[19] LUO P, YIN PY, HUA R, et al. A large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma[J]. Hepatology, 2018, 67(2):662
[20] SUN X, SHI J, LI R, et al. SWATH-MS2&1: development and validation of a pseudotargeted lipidomics method for the analysis of glycerol esters in milk[J]. J Agric Food Chem, 2022, 70(10):3331


