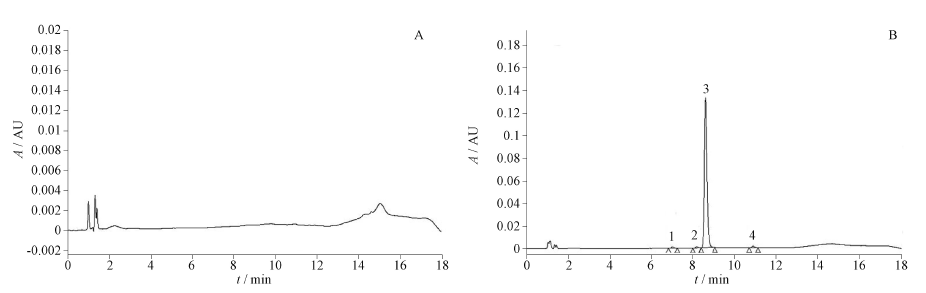
Objective: To determine seven impurities in oxytocin for injection and investigate the limit values. Methods: HPLC and principal component self-control with correction factor were adopted. The determination was performed on a Waters Xbridge C18 column(150 mm×4.6 mm, 5 μm). The mobile phase consisted of 0.1 mol·L-1 dihydrogen phosphate solution (adjusted to pH 5.4)-acetonitrile (90∶10, phase A), and acetonitrile (phase B) with gradient elution at a flow rate of 1.5 mL·min-1. The column temperature was maintained at 32 ℃, and the detection wavelength was set at 220 nm. The injection volume was 100 μL. The linear equations of oxytocin, impurities Ac-Oxy, Oxy[Glu4], Oxy[+Gly10], Oxy[-NH2], Oxy[trisulfide], Oxy[cis-dimer] and Oxy[trans-dimer] were drawn. The correction factors of each impurity related to oxytocin were calculated by slope. The contents of impurities in 3 batches of oxytocin for injection were determined and compared with the results of impurity reference method. Results: The limits of quantification for seven impurities were 2.75-5.66 ng, while the detection limits were 1.38-2.83 ng. The linear ranges of seven impurities were 0.03-3.40 μg·mL-1 with good linearity(r>0.999). The correction factors of Ac-Oxy, Oxy[Glu4] and Oxy[-NH2] were 1.1, while the correction factors of Oxy[+Gly10] and Oxy[trisulfide] were 1.2 and 0.9, respectively. The correction factors of Oxy[cis-dimer] and Oxy[trans-dimer] were both 1.3. The seven impurities were determined in 3 batches of samples by principal component self-control with correction factor. The contents of impurity Ac-Oxy were 0.96%,0.93% and 1.01%, respectively. The contents of impurity Oxy[Glu4] were 0.07%, 0.06% and 0.08%, respectively. The contents of impurity Oxy[+Gly10] were 0.07%, 0.04% and 0.04%, respectively. The contents of impurity Oxy[-NH2] were 0.09%, 0.05% and 0.07%, respectively. The contents of impurity Oxy[trans-dimer] were 0.27%, 0.18% and 0.22%, respectively. The maximum single impurity contents were 0.18%-0.19%, while the total impurity contents were 1.88%-2.06%. Compared the results measured by principal component self-control with correct factor method and the impurity reference method, there was no significant difference between two methods (p>0.05). Conclusion: The method is proved to be simple, repeatable and accurate for the content determination of related substances in oxytocin for injection.
LIU Ping, FAN Jun-pei, GU Jian-qin, SUN Jie, DOU Xiu-xiu, TANG Li-ming
. Determination of related substances in oxytocin for injection by HPLC-principal component self-control with correction factor[J]. Chinese Journal of Pharmaceutical Analysis, 2024
, 44(4)
: 671
-677
.
DOI: 10.16155/j.0254-1793.2024.04.14
[1] 黄青, 陆益红, 史清水, 等.高效液相色谱法测定缩宫素注射剂含量及其与生物效价测定法比较和应用[J]. 药物分析杂志, 2015, 35(6):1115
HUANG Q, LU YH, SHI QS, et al. HPLC determination of oxytocin injection and comparison with biological titer measurement and its applications[J]. Chin J Pharm Anal, 2015, 35(6):1115
[2] 陈建国, 陆益红, 黄青, 等. 缩宫素的制备及其质量分析研究进展[J]. 中国生化药物杂志, 2012, 33(5):698
CHEN JG, LU YH, HUANG Q, et al. Research advances in the preparation and quality control of oxytocin[J]. Chin J Biochem Pharm, 2012, 33(5):698
[3] 朱俊, 黄臻辉, 朱建伟. 缩宫素生产工艺的工业化进展[J]. 中国医药工业杂志, 2018, 49(7):910
ZHU J, HUANG ZH, ZHU JW, et al. Industrialization process of oxytocin production[J]. Chin J Pharm, 2018, 49(7):910
[4] 王振平, 贾存宇, 李晓倩, 等. 一种缩宫素及至少十种杂质的分离方法和应用:中国, CN111912917B[P]. 2022-03-15
WANG ZP, JIA CY, LI XQ, et al. Oxytocin and Ten Imupurities Separation Method and Application: China, CN111912917B[P]. 2022-03-15
[5] 中华人民共和国药典 2020年版. 二部[S]. 2020: 1790
ChP 2020. VolⅡ[S]. 2020: 1790
[6] USP 43-NF 38[S]. 2023: 3366
[7] EP 11.0[S]. 2023: 3617
[8] 黄青, 陆益红, 史清水, 等. 缩宫素注射液的有关物质研究[J]. 药物分析杂志, 2011, 31(10):1856
HUANG Q, LU YH, SHI QS, et al. HPLC determination of oxytocin injection and its related substances[J].Chin J Pharm Anal, 2011, 31(10):1856
[9] 付晓平, 高剑, 钟国庆, 等. 一种卡贝缩宫素杂质Gly9-OH的制备方法:中国, CN112010945B[P]. 2022-09-16
FU XP, GAO J, ZHONG GQ, et al. Carbetocin Impurity Gly9-OH Preparation Method: China, CN112010945B[P]. 2022-09-16
[10] 王振平, 李妍哲, 李娟, 等. 一种缩宫素及其8种差向异构体的分离方法及应用: 中国, CN111830154B[P]. 2022-05-24
WANG ZP, LI YZ, LI J, et al. Oxytocin and Eight Epimer Separation Method and Application: China, CN111830154B[P]. 2022-05-24
[11] 黄岭, 朱墨, 胡金涛, 等. 一种缩宫素原料药有关物质的高效液相色谱分离方法: 中国, CN113252807B[P]. 2022-05-06
HUANG L, ZHU M, HU JT, et al. HPLC Determination of Oxytocin for Injection and Its Related Substances: China, CN113252807B[P]. 2022-05-06
[12] 肖亭, 王晨, 姚尚辰, 等. HPLC校正因子法在药物分析中的应用[J]. 药学学报, 2020, 55(12):2854
XIAO T, WANG C, YAO SC, et al. Application of an HPLC correction factor method in pharmaceutical analysis[J]. Acta Pharm Sin, 2020, 55(12):2854
[13] GPH3-1化学药物杂质研究的技术指导原则[S]. 2005: 9
GPH3-1 Technical Guidelines for the Study of Impurities in Chemical Drugs[S]. 2005: 9
[14] 姜雄平, 李慧义, 陈桂良, 等. 药品标准中有关物质HPLC测定的几点意见[J]. 中国药品标准, 2013, 14(3):163
JIANG XP, LI HY, CHEN GL, et al. Suggestions on HPLC determination of drug related substances[J]. Drug Stand China, 2013, 14(3):163
[15] 余振喜, 庾莉菊, 黄海伟, 等. 浅谈HPLC法测定有关物质时已知杂质的计算方法[J]. 中国药品标准, 2010, 11(4):278
YU ZX, YU LJ, HUANG HW, et al. Discussion on the calculation methods of the known impurities in related substances determined by HPLC[J]. Drug Stand China, 2010, 11(4):278


