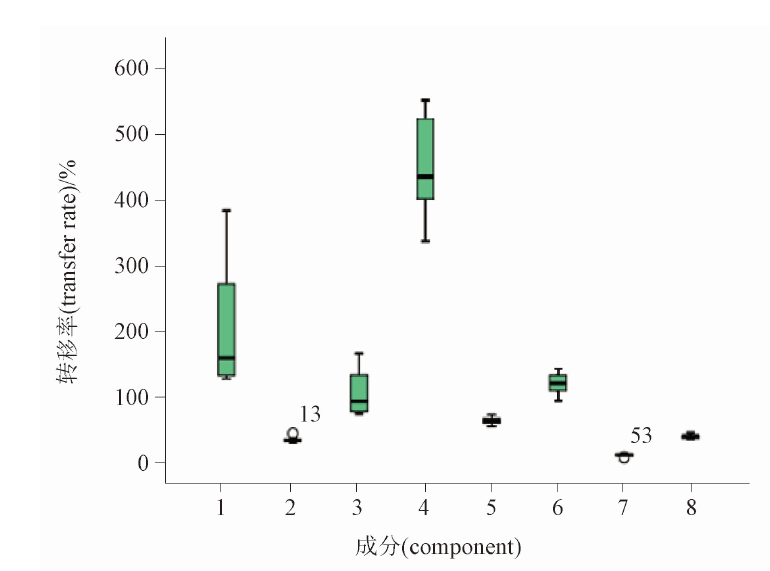
Objective: To establish a method for simultaneous determination of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, cynarin, galuteolin, isochlorogenic acid B, isochlorogenic acid A, isochlorogenic acid C in Shanjujiangya capsules by HPLC and analyze compositional change of Chrysanthemi Flos combined with law of quantity transfer. Methods: The analysis was performed on Waters Symmetry C18 column (250 mm×4.6 mm,5 μm), with mobile phase composed of acetonitrile -0.1% phosphoric acid solution at a flow rate of 1.0 mL·min-1 in gradient elution mode. The column temperature was 30 ℃ and the detection wavelength was 328 nm. The transfer rates of the above eight components were used as the indexes for quality evaluation to study the quantity value of transfer rule from the decoction piece to the extracting solution. Results: The results showed that the determination of eight components manifested a good linear relationship in the range of mass concentration (r>0.999 9), the average recoveries of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, cynarin, galuteolin, isochlorogenic acid B, isochlorogenic acid A, isochlorogenic acid C were 98.3%-101.9%,with RSDs of 0.066%-0.64%. The contents of the above 8 components in 3 samples were 0.257-0.279 mg·g-1, 0.629-0.650 mg·g-1, 0.402-0.476 mg·g-1, 0.454-0.539 mg·g-1, 1.118-1.278 mg·g-1, 0.653-0.740 mg·g-1, 0.659-0.706, 1.138-1.167 mg·g-1, respectively. Conclusion: The HPLC method established in this study is simple, repeatable and stable. The analysis of quantity transfer provides data support for the establishment of content methods and the formulation of limits. This study can provide basis for quality control method of Shanjujiangya capsules.
[1] 中华人民共和国药典2020年版.一部[S]. 2020:292
ChP 2020.Vol Ⅰ[S]. 2020:292
[2] 吴俊平,陈淑敏,杨光,等.基于HPLC多指标成分定量控制联合化学计量学的山菊降压胶囊质量评价研究[J]. 现代药物与临床,2021,36(10):2039
WU JP, CHEN SM, YANG G, et al. Study on quality evaluation of ShanjuJiangya capsules based on HPLC multi-index component quantitative control combined with stoichiometry [J]. Mod Med Clin, 2021, 36(10):2039
[3] 赵倩,黄维,彭成,等.中药多维配伍探索及创新药物发现[J]. 中华中医药杂志,2022,37(6):3298
ZHAN Q, HUANG W, PENG C, et al. Exploration of multi-dimensional compatibility of traditional Chinese medicine and discovery of innovative drugs [J]. China J Tradit Chin Med, 2022, 37(6):3298
[4] 吕玥,汤湧,李立言,等.中药复方制剂质量控制体系的研究进展[J]. 中国中西医结合外科杂志,2020,26(1):170
LÜ Y, TANG Y, LI LY, et al. Research progress of quality control system of traditional Chinese medicine compound preparation [J]. Chin J Surg Integr Tradit West Med, 2020, 26(1):170
[5] 邓启荣.中药复方质量标准研究的主要策略分析[J]. 中国药物经济学,2015,10(S2):47
DENG QR. Analysis of the main strategies in the study of the quality standard of traditional Chinese medicine compound [J]. China Pharmacoecon, 2015, 10(S2):47
[6] 张伟,吴瑞,常相伟,等.基于HPLC特征图谱结合化学计量学的菊花特征标志物的研究[J]. 天然产物研究与开发,2022,34(8):1289
ZHANG W, WU R, CHANG XW, et al. Study on characteristic markers of Chrysanthemum based on HPLC chromatogram combined with stoichiometry [J]. Nat Prod Res Dev, 2022, 34(8):1289
[7] 王月茹,谢伟.HPLC法测定药用菊花中10个主要化学成分的含量[J]. 世界中医药,2016,11(12):2778
WANG YR, XIE W. Determination of 10 main chemical components in Chrysanthemum by HPLC [J]. World Tradit Chin Med, 2016, 11(12):2778
[8] 宋程,陈存武,孙传伯,等.反相色谱法测定菊花中咖啡酸衍生物和黄酮类成分[J]. 中成药,2020,42(8):2084
SONG C, CHEN CW, SUN CB, et al. Determination of caffeic acid derivatives and flavonoids in chrysanthemum by reversed-phase chromatography [J]. China Tradit Pat Med, 2020, 42(8):2084
[9] 汤珺, 袁铭铭, 周国平,等. HPLC法同时测定小儿感冒颗粒中12个成分的含量[J]. 药物分析杂志,2022,42(12):2179
TANG J, YUAN MM, ZHOU GP, et al. Simultaneous determination of twelve chemical compositions in Xiaoer Ganmao granules by HPLC [J]. Chin J Pharm Anal, 2022, 42(12):2179
[10] 杨利民,张永刚,林红梅,等.中药材质量形成理论与控制技术研究进展[J]. 吉林农业大学学报,2012,34(2):119
YANG LM, ZHANG YG, LIN HM, et al. Research progress on quality formation theory and control technology of Chinese medicinal materials [J]. J Jilin Agric Univ, 2012, 34(2):119
[11] 赵小勤,许莉,李庆,等.水煎煮条件下知母新芒果苷和芒果苷成分转化关系研究[J]. 中国药物评价,2022,39(4):304
ZHAO XQ, XU L, LI Q, et al. Study on the transformation relationship between nemangiferin and mangiferin components in decocted water [J]. Chin J Pharm Eval, 2022, 39(4):304
[12] 王丽灵, 庞会明, 郑啸,等. 山萸肉炮制前后 11 种成分的变化[J]. 药物分析杂志,2016,36(4):624
WANG LL, PANG HM, ZHEN X, et al. Quantitative analysis of eleven active constituents in crude and processed Cornus officinalis [J] . Chin J Pharm Anal, 2016, 36(4):624
[13] 张雪,郭东晓,崔伟亮,等.金银花炮制前后咖啡酰奎宁酸类成分变化[J]. 中国药学杂志,2022,57(16):1337
ZHANG X, GUO DX, CUI WL, et al. Changes of caffiacyl quinone composition before and after processing of honeysuckle [J]. Chin J Pharm, 2022, 57(16):1337
[14] 吴笛,郭纯.HPLC法同时测定不同品种、产地、商品规格杭菊中9种成分[J]. 中成药,2021,43(5):1231
WU D, GUO C. Simultaneous determination of 9 components in different varieties, origin and commodity specifications of Hangju by HPLC [J]. Chin Tradit Pat Med, 2021, 43(5):1231
[15] 牛广东.不同产地杭白菊中绿原酸、木樨草苷、异绿原酸含量分析[J]. 河南大学学报(医学版),2022,41(2):99
NIU GD. Analysis of chlorogenic acid, luteolin and isochlorogenic acid in chrysanthemum from different habitats [J]. J Henan Univ (Med Ed), 2022, 41(2):99
[16] 李辉,逯桃桃,陈佳,等.金银花绿原酸提取方法研究[J]. 分析测试技术与仪器,2022,28(4):416
LI H, LU TT, CHEN J, et al. Study on extraction method of chlorogenic acid from Honeysuckle [J]. Anal Test Technol Instrum, 2022, 28(4):416
[17] 朱帮会,李银,马雪,等.高效液相色谱法同时测定醒脾养儿颗粒中6种咖啡酰奎宁酸类成分含量[J]. 中国药业,2022,31(19):59
ZHU BH, LI Y, MA X, et al. Simultaneous determination of six caffeoyl quinic acids in Xingpi Yanger granules by high performance liquid chromatography [J]. China Pharm, 2022, 31(19):59
[18] 王赛,谢逸轩,田硕,等.中药组分-药性-药效关系探讨[J]. 中药药理与临床,2023,39(4):125
WANG S, XIE YX, TIAN S, et al. Study on the relationship between TCM components, drug properties and efficacy [J]. Pharmacol Clin Chin Mater Med, 2023, 39(4):125
[19] 王乃康.中药制剂生产工艺存在的问题和对策探讨[J]. 内蒙古中医药,2022,41(6):122
WANG NK. Discussion on the problems and countermeasures of the production technology of traditional Chinese medicine [J]. Inner Mongolia Tradit Chin Med, 2022, 41(6):122
[20] 刘涛,苟小军,郭晓恒,等.基于中成药工艺与质量控制的再评价模式商建[J]. 中草药,2011,42(10):1873
LIU T, GOU XJ, GUO XH, et al. Construction of reevaluation model based on process and quality control of Chinese patent medicine [J]. Chin Tradit Herb Drugs, 2011, 42(10):1873



