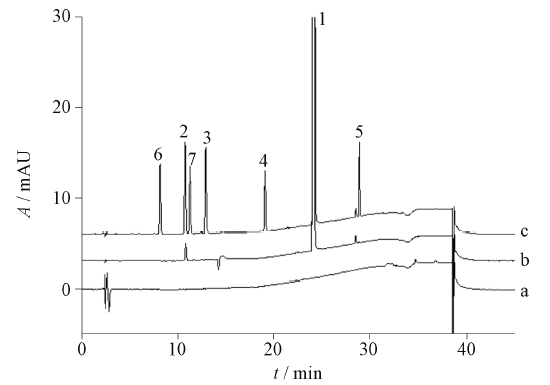
目的: 建立高效液相色谱同时测定N-芴甲氧羰基-O-叔丁基-L-苏氨酸中6个特定杂质的方法。方法: 采用YMC Triart C18(250 mm×4.6 mm,3 μm)色谱柱,以0.1%三氟乙酸水溶液为流动相A,以0.1%三氟乙酸乙腈溶液为流动相B,流速1.0 mL·min-1,梯度洗脱,检测波长265 nm,柱温30 ℃,进样体积10 μL。结果: N-芴甲氧羰基-O-叔丁基-L-苏氨酸与相邻杂质峰的分离良好;6个杂质分离度均大于1.5;且在相应质量浓度范围内呈现良好的线性关系(r≥0.999);6个杂质检测限和定量限分别约为0.03 μg·mL-1和0.06 μg·mL-1;6个杂质的平均回收率(n=9)在97.6%~98.8%范围内。3批N-芴甲氧羰基-O-叔丁基-L-苏氨酸测定结果显示,杂质1的含量<0.2%,杂质4的含量<0.1%,其他4种杂质未检出,总杂含量<1%。结论: 本方法分离度好,灵敏度高,专属性强,适用于N-芴甲氧羰基-O-叔丁基-L-苏氨酸中有关物质的检测。
Objective: To establish an HPLC method for the determination of six related substances in N-fluorenylmethoxycarbonyl-O-tert-butyl-L-threonine. Methods: The analysis was conducted on YMC Triart C18(250 mm×4.6 mm, 3μm) column, the mobile phase was consisted with 0.1% trifluoroacetic acid in water(A) and 0.1% trifluoroacetic acid in acetonitrile(B)at the flow rate of 1.0 mL·min-1. The column temperature was set 30 ℃, the detection wavelength was 265 nm and the injection volume was 10 μL. Results: N-Fluorenylmethoxycarbonyl-O-tert-butyl-L-threonine had good separation from the adjacent impurity peaks; The resolution of impurity1- 6 was greater than 1.5, and showed a good linear relationship (r≥0.999) in the corresponding mass concentration range, the detection limit of impurity 1-6 was 0.03 μg·mL-1, and the quantitative limit was 0.06 μg·mL-1, the average recovery rate (n=9) of impurity 1-6 was in the range of 97.6%-98.8%. The results of the three batches of N-fluorenylmethoxycarbonyl-O-tert-butyl-L-threonine showed that the contents of impurity 2, impurity 4 were <0.2% and <0.1%, respectively, and the other four impurities were not detected, and content of the total impurity was <1%. Conclusion: This method has good resolution, high sensitivity and strong specificity, and is suitable for the determination of related substances in N-fluorenylmethoxycarbonyl-O-tert-butyl-L-threonine.
[1] 高云龙.多肽药物合成和纯化方法发展现状[J]. 医药前沿, 2022, 12(14):22
GAO YL. Development status of peptide drug synthesis and purification methods[J]. J Front Med, 2022, 12(14):22
[2] 牛潇爽,胡争,周秀曼,等.多肽药物在肿瘤免疫治疗中的研究进展[J]. 中国肿瘤临床, 2023, 50(6):272
NIU XS, HU Z, ZHOU XM, et al. Research progress on peptide drugs in cancer immunotherapy[J]. Chin J Clin Oncol, 2023, 50(6):272
[3] 郑龙, 田佳鑫, 张泽鹏,等.多肽药物制备工艺研究进展[J]. 化工学报, 2021, 72(7):3538
ZHENG L, TIAN JX, ZHANG ZP, et al. Progress on pharmaceutical engineering of peptide-based drugs[J]. CIESC J, 2021, 72(7):3538
[4] 徐晓寒,吴闻哲.多肽类药物微球注射制剂的研究进展[J]. 中国医药工业杂志, 2014, 45(10):985
XU XH, WU WZ. Progress in injectable microsphere formulations of peptide drugs[J]. Chin J Pharm, 2014, 45(10):985
[5] 顾玲玲, 吴忠虹, 尹霞, 等.多肽类药物长效微球制剂仿制药研发要点浅析[J]. 中国医药工业杂志, 2021, 52(11):1436
GU LL, WU ZH, YIN X, et al. Introduction to key points for development of generic long-acting microsphere preparations of peptide drugs[J]. Chin J Pharm, 2021, 52(11):1436
[6] 戴佳, 赵巧君, 童玥.合成多肽药物质量控制研究探讨[J]. 药物生物技术, 2023, 30(1):69
DAI J, ZHAO QJ, TONG Y. Quality control of synthetic peptide drugs[J]. Pharm Biotechnol, 2023, 30(1):69
[7] 林龙, 姜素云, 汤新强.多肽类药物体外合成方法的研究进展[J]. 大连医科大学学报, 2014, 36(2):177
LIN L, JIANG SY, TANG XQ. Recent research progress in the methods of in vitro synthesis of peptide drugs[J]. J Dalian Med Univ, 2014, 36(2):177
[8] 胡玉玺, 蒋煜, 韩天娇.制备工艺和过程控制对合成多肽药物有关物质的影响[J]. 中国新药杂志, 2017, 26(18):2143
HU YX, JIANG Y, HAN TJ. Effects of manufacturing process and process control on related substances of synthetic peptide drugs[J]. Chin J New Drugs, 2017, 26(18):2143
[9] 吴一凡, 刘福利.多肽固相合成中起始物料的控制策略[J]. 中国新药杂志, 2022, 31(10):937
WU YF, LIU FL. The control strategy of starting materials used in solid-phase peptide synthesis[J]. Chin J New Drugs, 2022, 31(10):937
[10] 王鹏.合成多肽药物的合成工艺中关键问题分析[J]. 中国新药杂志, 2010, 19(2):102
WANG P. Analysis of key problem on synthesis of synthetic peptide drugs[J]. Chin J New Drugs, 2010, 19(2):102
[11] EGGENI. Control strategies for synthetic therapeutic peptide APIs part Ⅰ: analytical considerations[J]. Biopharm Intern, 2014, 27(3):16
[12] 国家药品监督管理局.化学合成多肽药物药学研究技术指导原则[S]. 2023
National Medical Products Administration. Technical Guidelines for Research in the Synthetic Peptide Drugs[S]. 2023
[13] 林凤强, 李铁健, 杨欣茹, 等.HPLC法HILIC模式测定生长抑素中对映异构体D-Ala1-生长抑素的含量[J]. 药物分析杂志, 2022, 42(3):419
LIN FQ, LI TJ, YANG XR, et al. Determination of enantiomeric purity D-Ala1-somatostatin in somatostatin by HILIC HPLC[J]. Chin J Pharm Anal, 2022, 42(3):419
[14] 李茜, 梅芊, 刘英.醋酸奥曲肽原料及其制剂的杂质分析[J]. 药物分析杂志, 2017, 37(3):492
LI Q, MEI Q, LIU Y. Analysis of impurities in octreotide acetate and its injection[J]. Chin J Pharm Anal, 2017, 37(3):492
[15] 忻余, 徐康森.高效液相色谱法测定尿多酸肽注射液中小分子肽的含量[J]药物分析杂志, 2001, 21(3):191
XIN Y, XU KS. HPLC determination of peptide's molecular weight in niaoduosuantai injection[J]. Chin J Pharm Anal, 2001, 21(3):191
[16] 邱芊, 陈永森.固相多肽合成中氨基酸保护的研究进展[J]. 化工时刊, 2005, 19(6):56
QIU Q, CHEN YS. The Development of amino protecting groups using in solid-phase peptide synthesis[J]. Chem Ind Times, 2005, 19(6):56
[17] 林琳, 夏立钧, 许旭, 等.大环糖肽抗生素键合相高效液相色谱法拆分7种氨基带保护基的氨基酸对映体 [J]. 色谱, 2006,24(2):144
LIN L, XIA LJ, XU X, et al. Separation of enantiomers of amino acid derivatives by high performance liquid chromatography on teicoplanin chiral stationary phase[J]. Chin J Chromatogr, 2006, 24(2):144
[18] 杨欣茹, 李铁健, 王金迪, 等.反相高效液相色谱法测定Fmoc-L-His(Trt)-OH中光学异构体Fmoc-D-His(Trt)-OH的含量[J]. 中南药学, 2023, 21(8):2170
YANG XR, LI TJ, WANG JD, et al. Determination of optical isomer Fmoc-D-His(Trt)-OH in Fmoc-L-His(Trt)-OH by RP-HPLC[J]. Cent South Pharm, 2023, 21(8):2170



