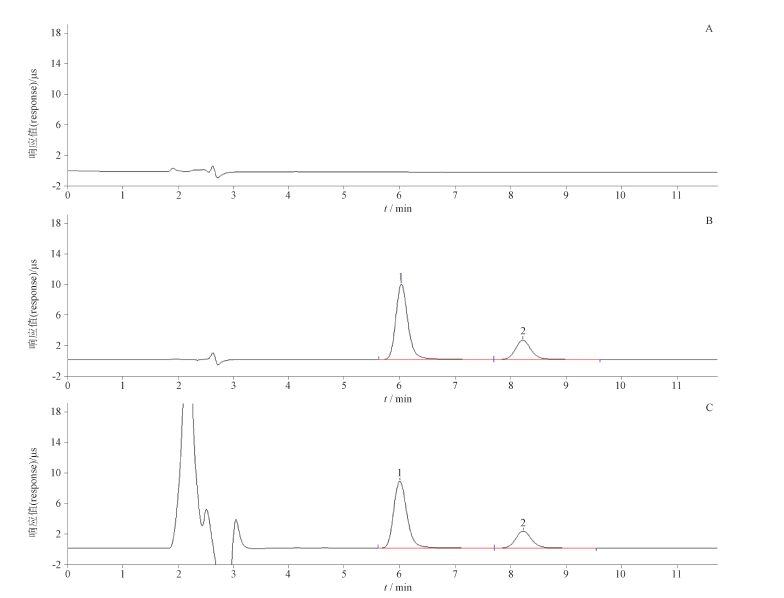
目的:建立离子色谱法测定人血白蛋白制品中辛酸钠的含量。方法:样品加淋洗液[甲醇-乙腈-1.0 mmol·L-1盐酸(20:42:38)]沉淀蛋白,离心取上清液,过滤后直接进样,以庚酸为内标。采用Dionex InPacTM NS1分析柱(250 mm×4 mm,10 μm)与Dionex InPacTM NG1保护柱(35 mm×4 mm,10 μm),流速1.0 mL·min-1,ASRS 300 膜抑制器,化学抑制,再生液为 5 mmol·L-1四丁基氢氧化钠溶液,电导检测器检测,柱温 30 ℃,进样量 25 μL。结果:辛酸峰与内标峰间的分离度>1.5;辛酸钠在0.38~2.52 mmol·L-1范围内线性关系良好,r=0.999 5(n=6);重复性试验的RSD为1.1%(n=6);平均加样回收率为97.4%,RSD为1.8%(n=9);定量限与检测限分别为0.19 nmol与0.09 nmol;国内外7家企业20批人血白蛋白制品中辛酸钠含量范围为0.073~0.163 mmol·g-1。结论:本研究建立的方法操作简便易行,结果准确,灵敏度高,重复性好,可用于人血白蛋白制品中辛酸钠含量的测定,为其质量控制提供方法保证。
[1] 赵大辉,王桂茹,陈秀文,等. 人血白蛋白的生理功能及其应用护理研究[J]. 护理研究,2011,25(5):380
ZHAO DH, WANG GR, CHEN XW, et al. Nursing research on physiological function of human albumin and its application[J]. Chin Nurs Res, 2011, 25(5):380
[2] 欧阳生珀,童荣生.人血白蛋白的合理应用概述[J]. 中国医院药学志,2021,41(4):425
OUYANG SP, TONG RS. Overview of the rational use of human albumin[J]. Chin J Hosp Pharm, 2021, 41(4):425
[3] 徐明明,赵沁,周勤,等.人血白蛋白中蛋白聚合物的研究[J]. 中国药事, 2021,35 (11):1246
XU MM, ZHAO Q, ZHOU Q, et al. Research of protein aggregates in human albumin[J]. Chin Pharm Aff, 2021, 35 (11):1246
[4] 丁锐,纪宏,余立.人血白蛋白不溶性微粒检测方法的建立及测定结果[J]. 中国医院药学杂志,2010,30(14):1197
DING R, JI H, YU L. Establishment and the results of testing method for measuring the particulate matter in human albumin[J]. Chin J Hosp Pharm, 2010, 30(14):1197
[5] 鲁松,杨萍,杨黎.人血白蛋白注射液的药品评价研究进展[J]. 中国药物警戒,2022, 19 (2):228
LU S, YANG P, YANG L, et al. Research progress in human serum albumin injections[J]. Chin J Pharm, 2022, 19 (2):228
[6] 庞赛,庞发根,刘晶晶,等.火焰光度法测定人血白蛋白中钠离子含量的不确定度评定[J],中国药物评价,2021,38(3):196
PANG S, PANG FG, LIU JJ, et al. Evaluation of measurement uncertainty for the etermination of sodium ion content of human albumin by flame atomic absorption spectrometry[J]. Chin J Drug Eval, 2021, 38(3):196
[7] 王敏力,肖林,周倩,等.首批人血白蛋白国家参考品的研制[J]. 中国药学杂志,2017,52(6):480
WANG ML, XIAO L, ZHOU Q, et al. Preparation of the first Chinese national standard for human albumin[J]. Chin Pharm J, 2017, 52 (6):480
[8] 窦姿, 赵小洁,张齐明,等.人血白蛋白生产过程中多聚体含量的检测[J]. 中国输血杂志,2021,34(8): 909
DOU Z, ZHAO XQ, ZHANG QM, et al. Determnation of polymer content in human albumin production process[J]. Chin J Blood Trans, 2021, 34(8): 909
[9] 陈学奎,邹澄川,杨泽蓉.离子排斥色谱法测定白蛋白中的辛酸钠含量[J]. 中国输血杂志,2000,13(4):242
CHEN XQ, ZOU CC, YANG ZR. Determination the content of sodium caprylate in albumin by ion exclusion chromatography[J]. Chin J Blood Trans, 2000, 13(4):242
[10] KHLYBOVA EV, KORMSHCHIKOVA ES, DROBKOVA AV, et al. A chromatographic method for assay of sodium caprylate in the formulation [J]. Pharm Chem J, 2017, 51(3):235
[11] 王文杰,吴翠,宋大章,等.气相色谱法测定人血白蛋白辛酸钠含量的方法优化[J] . 生物化工,2022,8(4):99
WANG WJ,WU C, SONG DZ, et al. Optimization of gas chromatography for determination of sodium caprylate in human albumin[J]. Biol Chem Enging, 2022, 8(4):99
[12] 中华人民共和国药典2020 年版.三部[S]. 2020:495
ChP 2020.Vol Ⅲ[S]. 2020:495
[13] 赵明芬,王霞,李荣贞,等.气相色谱法测定人血白蛋白半成品中辛酸钠含量[J]. 中国药品标准,2017,18(1):37
ZHAO MF, WANG X, LI RZ, et al. Determination of sodium caprilate in the human albumin semi-finished product by gas chromatography[J]. Drug Stand China, 2017, 18(1):37
[14] 仲立军,刘欣晏,李庆英,等. 人血白蛋白中辛酸钠的含量测定[J]. 中国医院药学杂志,2006,26(11):1443
ZHONG LJ, LIU XY, LI QY, et al. Determination of sodium caprilate in the human albumin[J]. Chin J Hosp Pharm,2006,26(11):1443
[15] 蒋翠岚,王立娜,韩继红,等.柱前衍生-反相高效液相色谱法测定人血白蛋白中辛酸钠含量[J]. 分析化学,2000,38(3):425
JIANG CL, WANG LN, HAN JH, et al. Determination of sodium caprilate in the human albumin by pre-column derivatization-reversed-phase high performance liquid chromatography[J]. Chin J Anal Chem, 2000, 38(3):425
[16] 卢胜娟,刘艳花,陈金利.反相高效液相色谱法测定人血白蛋白制品中辛酸钠含量[J]. 河北企业,2015(4):95
LU SJ, LIU YH, CHEN JL. Determination the content of sodium caprylate in human blood albumin products by reversed-phase high performance liquid chromatography[J]. Hebei Qiye, 2015(4):95
[17] 倪永碧,谷兰. 酸碱滴定法定量测定白蛋白制品中辛酸钠含量[J]. 中国输血杂志, 1994,7(3):123
NI YB, GU L. Quantitative determination of octanoate content in albumin product by acid base titration[J]. Chin J Blood Trans, 1994, 7(3):123
[18] 李荣贞,李庆英,王彬.两种定量测定人血白蛋白中辛酸钠含量方法的比较[J]. 药学研究,2014,33(2):85
LI RZ, LI QY, WANG B. Study on two methods of sodium caprilate assay determination in human serum albumin[J]. Pharm J Res, 2014, 33(2):85
[19] 王娜妮,费莹,王绪平,等. 离子色谱在中药分析中的应用研究[J]. 食品安全质量检测学报,2015,6(12):4883
WANG NN, FEI Y, WANG XP, et al. Application studies of the analysis of traditional Chinese medicine by ion chromatography[J]. J Food Saf Qual, 2015, 6(12): 4883
[21] 张文博,吴闯.三氯甲烷对卤虫的急性毒性研究[J]. 当代化工,2015,44(9):2264
ZHANG WB, WU C. Study on acute toxicity of chloroform on Artemia francisana[J]. Contemp Chem Ind,2015,44(9):2264