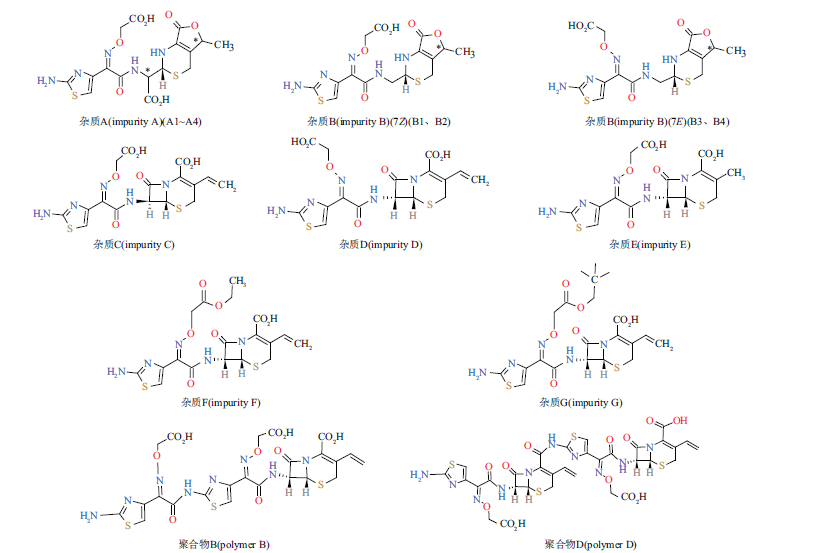
目的:改进头孢克肟颗粒有关物质的液相色谱测定方法。方法:使用高效液相色谱仪,选择YMC-Triart C18色谱柱(250 mm×4.6 mm,5 μm),以0.05 mol·L-1甲酸铵溶液(pH 4.7)-甲醇为流动相,流速1 mL·min-1,进行梯度洗脱,进样量为10 μL,检测波长为254 nm。结果:将该色谱条件应用于头孢克肟颗粒有关物质的检测,对比了本文方法与药典方法(含USP PF 2018版)中有关物质测定方法之间的差异,并完成了专属性、线性、准确度、精密度和耐用性等系统的方法学验证。药典方法均无法同时使主要降解杂质A1~A4或杂质B1~B4基线分离,且无法用于测定聚合物杂质B及聚合物杂质D。本文方法头孢克肟、各特定杂质之间的分离度均符合要求(R≥1.5),可同时检测并定量聚合物B及聚合物D,分离度优于药典方法。结论:本方法改进了头孢克肟、各杂质间的分离度,杂质检出个数更多,能准确定量各特定杂质,灵敏度较高,重复性较好,适用于头孢克肟的质量控制。
Objective: To improve the liquid chromatographic determination method of cefixime granules related substance. Methods: High performance liquid chromatography was used, YMC-Triart C18 column (250 mm×4.6 mm, 5 μm) was selected, 0.05 mol·L-1 ammonium formate solution (pH 4.7)-methanol was used as mobile phase, flow rate was 1 mL·min-1, and gradient washing was carried out.The injection volume was 10 μL. The detection wavelength was 254 nm. Results: This chromatographic condition was applied to the detection of cefixime granules. The differences between this method, the pharmacopeial method and the method of USP PF 2018 were compared, and the systematic methodological verification of specificity, linearity, accuracy, precision and durability were completed. Using pharmacopeial methods, baseline separation of degradation impurities A1~A4 or impurities B1~B4 cannot be reached, and current methods cannot be used to determine polymer B and polymer D. The method proposed in this article can make the resolution between cefixime and each specific impurities meet the requirements (R ≥1.5), and can detect and quantify polymer B and polymer D at the same time, and the resolution was better than the current method. Conclusion: This method improves the separation between cefixime and impurities, more impurities is detected and can accurate quantify specific impurities. This method has high sensitivity and good repeatability, and is suitable for the quality control of cefixime.
[1] 袁海龙,李仙义,王敬国,等.头孢克肟的药理与临床应用[J]. 中国医院药学杂志,1994,14(3):111
YUAN HL, LI XY, WANG JG, et al. Pharmacology and clinical application of cefixime[J]. Chin J Hosp Pharm,1994,14(3):111
[2] 王惠平.头孢克肟治疗泌尿生殖系统感染78例[J]. 现代中西医结合杂志,2007,16(17):2365
WANG HP. Cefixime was treated with 78 cases of genitourinary infection [J]. Mod J Integr Tradit Chin West Med, 2007,16(17):2365
[3] 邓鸣,陈宁周,李浩,等.国产头孢克肟口服固体制剂有关物质研究[J]. 中国抗生素杂志,2016,41(7):541
DENG M, CHEN NZ, LI H, et al. Studies on impurities in domestic oral solid preparation of cefexime[J]. Chin J Antibiot, 2016,41(7):541
[4] 李进,姚尚辰,尹利辉,等.头孢克肟原料及制剂的聚合物杂质分析[J]. 药学学报,2020,55(10):2442
LI J, YAO SC, YIN LH, et al. Analysis of polymer impurities in cefixime raw materials and preparations[J]. Acta Pharm Sin, 2020,55(10):2442
[5] USP PF 2018[S]. 2018:231
[6] 中华人民共和国药典2020年版.二部[S]. 2020:313
ChP 2020.Vol Ⅱ[S]. 2020:313
[7] USP 2023[S]. 2023: Cefixime for Oral Suspension
[8] JP 18[S]. 2021:674
[9] EP 11.0[S]. 2023:2239
[10] BP 2023[S]. 2023:473
[11] 边磊,宋敏,杭太俊,等.乙腈辅助电喷雾LC-MS/MS法分析头孢克肟中的有关物质[J]. 药物分析杂志,2010,30(5):872
BIAN L, SONG M, HANG TJ, et al. Identification of the related substances in cefixime by LC-MS/MS with acetonitrile aided electrospray ionization [J]. Chin J Pharm Anal,2010,30(5):872
[12] 张慧文,关倩明,林玲,等.RP-HPLC法测定头孢克肟的有关物质[J]. 中国抗生素杂志,2010,35(3):209
ZHANG HW, GUAN QM, LIN L, et al. Determination of related substances in cefixime by RP-HPLC [J]. Chin J Antibiot, 2010,35(3):209
[13] 李海霞,任进民,闫随朝,等. LC/MS/MS法测定人血浆中头孢克肟含量[J]. 中国抗生素杂志,2011,36(9):680
LI HX, REN JM, YAN SC, et al. Sensitive liquid chromatography-tandem mass spectrometry method for the determination of cefixime in human plasma[J]. Chin J Antibiot,2011,36(9):680
[14] 石海英,陈修毅,王金虎.分子排阻色谱法测定头孢克肟干混悬剂中高分子杂质的含量[J]. 中国抗生素杂志,2016,41(2):122
SHI HY, CHEN XY, WANG JH. Determination of high molecular mass impurities in cefixime for suspension by molecular-exclusion chromatography method [J]. Chin J Antibiot, 2016,41(2):122
[15] 陈鑫,王晨,竹张敏.头孢克肟合成工艺研究[J]. 中国抗生素杂志,2012,37(9):687
CHEN X, WANG C, ZHU ZM. Study on the synthesis of cefixime [J]. Chin J Antibiot, 2012, 37(9):687



