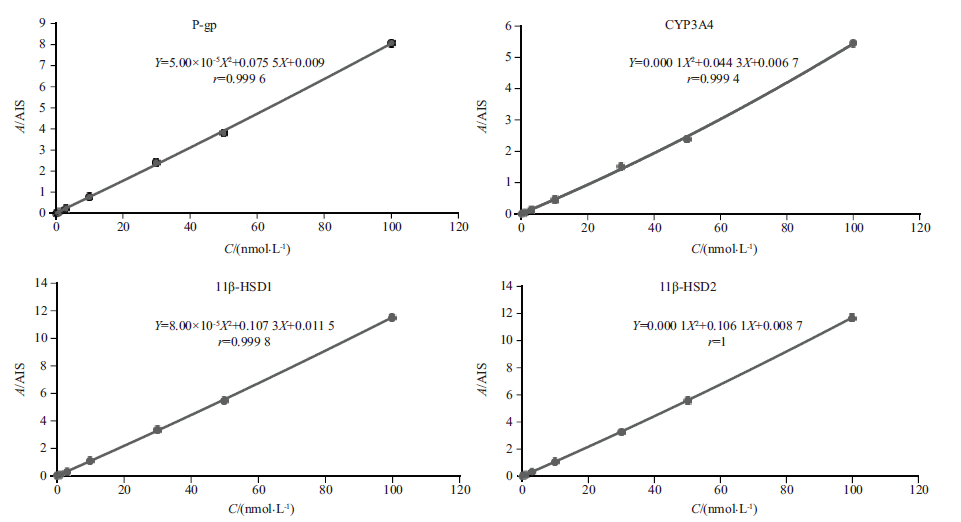
目的:建立LC-MS/MS方法测定中国孕妇胎盘中地塞米松相关代谢酶和转运体的丰度数据。方法:采用Shim-pack GISS-HP C18(100 mm×2.1 mm, 1.9 μm)色谱柱,柱前由0.2%甲酸-水(A)和0.2%甲酸-乙腈(B)组成的流动相,以0.2 mL·min-1的流速进行梯度洗脱,柱后以0.1 mL·min-1的流速加入0.5%乙二醇-乙腈(流动相C),采用ESI源正离子MRM模式进行定量分析。对所建立的方法进行方法学考察并对中国孕妇孕晚期胎盘中11β羟基类固醇脱氢酶1(11β-HSD1)、11β羟基类固醇脱氢酶2(11β-HSD2)、细胞色素P450 3A4酶(CYP3A4)和P-糖蛋白(P-gp)蛋白表达量进行定量分析。结果:建立的LC-MS/MS分析测定方法的线性范围为0.1~100 nmol·L-1(r>0.999);精密度和准确度结果均符合《中华人民共和国药典》对于生物样品分析方法学验证的要求(RSD≤15%),稳定性结果显示样品稳定性良好。11β-HSD2、11β-HSD1、CYP3A4和P-gp的蛋白丰度分别为(84.46±59.97) pmol·g-1、(11.44±3.73) pmol·g-1、 (8.83±2.78) pmol·g-1和(7.94±4.10) pmol·g-1。同时研究结果显示相关代谢酶和转运体在胎盘不同部位的分布没有显著差异,但中国人胎盘P-gp的丰度(7.94±4.10) pmol·g-1和白人(4.41±2.46) pmol·g-1相比存在显著性差异。结论:本研究建立的LC-MS/MS方法准确度和灵敏度高,适用于检测人类胎盘中地塞米松相关代谢酶和转运体的丰度值。
Objective: To establish an LC-MS/MS method for the determination of the abundance data of dexamethasone-related metabolic enzymes and transporters in the placenta of Chinese pregnant women . Methods: A Shim-pack GISS-HP C18 (100 mm×2.1 mm, 1.9 μm) chromatographic column was used, and the mobile phases were consisted of 0.2% formic acid-water (phase A) and 0.2% formic acid-acetonitrile (phase B) with gradient elution at a flow rate of 0.2 mL·min-1. The post-column phase C consisted of 0.5% ethylene glycol-acetonitrile was added at a flow rate of 0.1 mL·min-1, and the ESI source positive ion MRM mode was used for quantitative analysis. The established method was investigated methodologically and the expression levels of 11β hydroxysteroid dehydrogenase 1(11β-HSD1), 11β hydroxysteroid dehydrogenase 2(11β-HSD2), cytochrome P450 3A4 enzyme (CYP3A4) and P-glycoprotein protein (P-gp) were quantitatively analyzed in the placenta of Chinese pregnant women in the third trimester. Results: The established LC-MS/MS method had a linear range of 0.1-100 nmol·L-1(r >0.999). The precision and accuracy results met the requirements of the biological sample analysis method verification in Chinese Pharmacopoeia (RSD≤15%), and the stability results showed that the samples were stability. The protein abundance of 11β-HSD2, 11β-HSD1, CYP3A4 and P-gp were (84.46±59.97) pmol·g-1, (11.44±3.73) pmol·g-1, (8.83±2.78) pmol·g-1 and (7.94±4.10) pmol·g-1, respectively. Besides, the results of the study also showed that there was no significant difference in the distribution of related metabolic enzymes and transporters in different parts of the placenta. However, there was a significant difference in the abundance of P-gp in the placenta between Chinese people (7.94±4.10) pmol·g-1 and white people (4.41±2.46) pmol·g-1. Conclusions: The LC-MS/MS method established in this study has high accuracy and sensitivity and is suitable for detecting the abundance values of dexamethasone-related metabolic enzymes and transporters in human placenta.
[1] HALLMAN M, GLUMOFF V, RÄMET M. Surfactant in respiratory distress syndrome and lung injury[J]. Comp Biochem Physiol A Mol Integr Physiol, 2001, 129(1): 287
[2] SWEET DG, CARNIELLI V, GREISEN G, et al. European Consensus Guidelines on the management of respiratory distress syndrome—2019 update[J]. Neonatology, 2019, 115 (4):432
[3] EGERMAN RS, MERCER BM, DOSS JL, et al. A randomized, controlled trial of oral and intramuscular dexamethasone in the prevention of neonatal respiratory distress syndrome[J]. Am J Obstet Gynecol, 1998, 179(5): 1120
[4] THEOGARAJ E, JOHN CD, CHRISTIAN HC, et al. Perinatal glucocorticoid treatment produces molecular, functional, and morphological changes in the anterior pituitary gland of the adult male rat[J]. Endocrinology, 2005, 146(11):4804
[5] CHARNVISES S, FENCL MD, OSATHANONDH R, et al. Adrenal steroids in maternal and cord blood after dexamethasone administration at midterm[J]. J Clin Endocrinol Metab, 1985, 61(6):1220
[6] SCHMIDT AF, KEMP MW, MILAD M, et al. Oral dosing for antenatal corticosteroids in the Rhesus macaque[J]. PLoS One, 2019, 14(9):e0222817
[7] MARK PJ, WADDELL BJ. P-glycoprotein restricts access of cortisol and dexamethasone to the glucocorticoid receptor in placental BeWo cells[J]. Endocrinology, 2006, 147(11): 5147
[8] REBUFFAT AG, TAM S, NAWROCKI AR, et al. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist[J]. Mol Cell Endocrinol, 2004, 214(1-2): 27
[9] TOMLINSON ES, LEWIS DF, MAGGS JL, et al. In vitro metabolism of dexamethasone (DEX) in human liver and kidney: the involvement of CYP3A4 and CYP17 (17, 20 LYASE) and molecular modelling studies[J]. Biochem Pharmacol, 1997, 54(5): 605
[10] Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Anal Biochem, 1976, 72: 248
[11] NAKAMURA K, HIRAYAMA-KUROGI M, ITO S, et al. Large-scale multiplex absolute protein quantification of drug-metabolizing enzymes and transporters in human intestine, liver, and kidney microsomes by SWATH-MS: comparison with MRM/SRM and HR-MRM/PRM[J]. Proteomics, 2016, 16(15-16): 2106
[12] PRASAD B, UNADKAT JD. Optimized approaches for quantification of drug transporters in tissues and cells by MRM proteomics[J]. AAPS J, 2014, 16(4): 634
[13] ANOSHCHENKO O, PRASAD B, NERADUGOMMA NK, et al. Gestational age-dependent abundance of human placental transporters as determined by quantitative targeted proteomics[J]. Drug Metab Dispos, 2020, 48(9): 735
[14] JOSHI AA, VAIDYA SS, ST-PIERRE MV, et al. Placental ABC transporters: biological impact and pharmaceutical significance[J]. Pharm Res, 2016, 33(12): 2847
[15] LEE N, HEBERT MF, WAGNER DJ, et al. Organic cation transporter 3 facilitates fetal exposure to metformin during pregnancy[J]. Mol Pharmacol, 2018, 94(4): 1125



