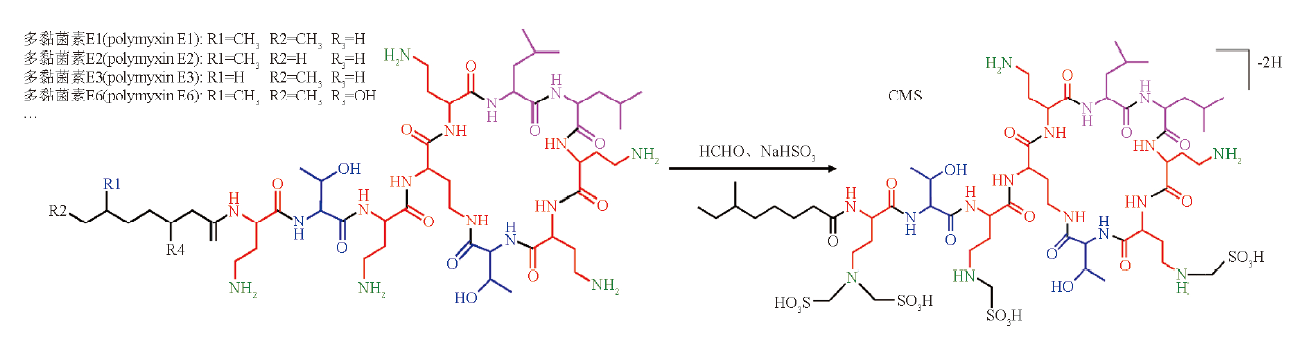
目的:建立二维液质联用方法确定多黏菌素E甲磺酸钠(CMS)杂质的结构及来源,进而用于药物质量控制研究。方法:一维系统:采用Acquity UPLC® Peptide CSH C18(150 mm×2.1 mm,1.7 μm)色谱柱,以磷酸盐缓冲液(7.8 g·L-1磷酸二氢钠,用1 mol·L-1氢氧化钠溶液调节pH至6.4)-乙腈(19∶1)为流动相A,磷酸盐缓冲液-乙腈(1∶1)为流动相B,梯度洗脱,流速0.3 mL·min-1,柱温30 ℃;二维系统:采用Acquity BEH C18柱(50 mm×2.1 mm,1.7 μm)色谱柱,以甲酸铵(A)-乙腈(B)为流动相,梯度洗脱,流速0.2 mL·min-1,柱温40 ℃,检测波长210 nm。质谱检测器采用ESI源,负离子扫描模式。结果:采用2D-LC-Q TOF MS法,推定了CMS中55个杂质的结构,并推测其主要来源为多黏菌素E1-I、多黏菌素E1-7MOA、多黏菌素E3及多黏菌素E6。结论:利用二维液质联用技术推定CMS中杂质峰的结构及来源,并用来评价不同厂家、生产工艺的原料药中杂质含量变化,有利于改进生产工艺,从源头控制药物质量。
Objective: To establish a suitable method to determine the structure and source of impurities of colistimethate sodium (CMS) for drug quality control studies. Methods: Frist-dimensional system: using Acquity UPLC® Peptide CSH C18(150 mm×2.1 mm, 1.7 μm) column, the mobile phase A was phosphate buffer (7.8 g·L-1 sodium dihydrogen phosphate, adjusted to pH 6.4 with 1 mol·L-1 sodium hydroxide)- acetonitrile (19∶1), the mobile phase B was phosphate buffer-acetonitrile (1∶1). Gradient elution was performed at a flow rate of 0.3 mL·min-1. The column temperature was 30 ℃. Second-dimensional system: the Acquity BEH C18 column (50 mm×2.1 mm, 1.7 μm) column was used with ammonium formate(A)-acetonitrile mixture as mobile phase with gradient elution. The flow rate was 0.2 mL·min-1. The column temperature was 40 ℃. The detection wave length was 210 nm. The ESI source was used in negative ion mode. Results: The 2D-LC-Q TOF MS method was used to infer the structure of the 55 impurities in CMS, and the main sources were polymyxin E1-I, polymyxin E1-7MOA, polymyxin E3 and polymyxin E6. Conclusion: The structure and source of impurities in CMS are determined by 2D-LC-Q TOF MS, and the changes in the content of impurities such as manufacturers and production processes are evaluated, which is conducive to improving the production process and controlling drug quality at the source.
[1] EL-SAYED AHMED MAE, ZHONG LL, SHEN C, et al. Colistin and its role in the Era of antibiotic resistance: an extended review (2000-2019)[J]. Emerg Microbes Infect, 2020, 9(1): 868
[2] DAGLA I, KARKOULA E, BAIRA E, et al. Analytical methodologies used for the determination of colistin in biological fluids. Is it still a challenge?[J]. J Pharm Biomed Anal, 2019, 164: 777
[3] VAN DEN BOSSCHE L, VAN SCHEPDEAL A, CHOPRA S, et al. Identification of impurities in polymyxin B and colistin bulk sample using liquid chromatography coupled to mass spectrometry[J]. Talanta, 2011, 83(5): 1521
[4] 张敏. 药物中有关物质检测方法研究进展及应用[J]. 广东化工, 2021, 48(4): 129
ZHANG M. Research advance and application of detection methods for related substances in drugs[J]. Guangdong Chem Ind, 2021, 48(4): 129
[5] 吴勇, 周梦漪, 薛春佳, 等. 多黏菌素E类似物的纯化和结构鉴定[J]. 中国抗生素杂志, 2018, 43(1): 59
WU Y, ZHOU MY, XUE CJ, et al. Purification and structure elucidation of colistin E analogues[J]. Chin J Antibiot, 2018, 43(1): 59
[6] EP 10.3. Vol Ⅲ[S]. 2020: 4941
[7] USP 43-NF 38. Vol Ⅲ[S]. 2020: 1148
[8] JP 18. VolⅠ[S]. 2021: 810
[9] European Medicines Agency. Assessment Report Polymyxin-Based Products; European Medicines Agency[EB/OL]. London, 2015 [2015-02-16]. https://www.ema.europa.eu/contact
[10] DAGLA I, TSARBOPOULOS A, GIKAS E. A novel validated injectable colistimethate sodium analysis combining advanced chemometrics and design of experiments[J]. Molecules, 2021, 26, 1546
[11] 刘丹, 李亮, 刘彩, 等. 甲磺酸多黏菌素E2组分的色谱分离及质谱裂解规律研究[J]. 质谱学报, 2018, 39(6): 653
LIU D, LI L, LIU C, et al. Chromatographic separation and fragmentation mechanism in electrospray ionization mass spectrometry for colistin B methanesulfonate[J]. J Chin Mass Spectrom Soc, 2018, 39(6):653
[12] LI J, MILNE RW, NATION RL, et al. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate[J]. J Antimicrob Chemother, 2004, 53(5): 837
[13] MERCIER T, TISSOT F, GARDIOL C, et al. High-throughput hydrophilic interaction chromatography coupled to tandem mass spectrometry for the optimized quantification of the anti-Gram-negatives antibiotic colistin A/B and its pro-drug colistimethate[J]. J Chromatogr A, 2014, 1369: 52
[14] NATION RL, VELKOV T, LI JT. Colistin and polymyxin B: are they like peas in a pod or chalk and cheese?[J]. Clin Infect Dis, 2014, 59(1): 88
[15] 钮晓淑, 胡昌勤, 常艳, 等. 多黏菌素类抗生素的研发沿革与现状[J]. 中国药师, 2021, 24(5): 936
NIU XS, HU CQ, CHANG Y, et al. History and status of development of polymyxin antibiotics[J]. China Pharm, 2021, 24(5): 936



