目的:建立固相萃取-高效液相色谱法(SPE-HPLC法)快速检测维生素D滴剂(软胶囊)中维生素D3有关物质反式维生素D3(杂质A)、7-去氢胆固醇(杂质B)、光甾醇3(杂质C)、异速甾醇3(杂质D)。方法:取内容物约1.2 g(约相当于维生素D3 1 548 IU),精密称定,置于试管中,加入异辛烷4 mL,涡旋混匀,采用固相萃取小柱进行净化,以正己烷-乙酸乙酯(85∶15)为洗脱剂,无水乙醇为解吸剂;采用Luna Silica(2)100 Å硅胶柱(150 mm×4.6 mm,3 μm),以正己烷-正戊醇(996.5∶3.5)为流动相,采用加校正因子主成分自身对照法计算维生素D3各杂质的含量。结果:前维生素D3与植物油分离度>1.5。维生素D3的线性范围为0.02~0.80 μg·mL-1,r=0.999 5;杂质A的线性范围为0.02~0.80 μg·mL-1,r=0.999 9;杂质B的线性范围为0.02~0.80 μg·mL-1,r=0.999 9;杂质C的线性范围为0.02~0.80 μg·mL-1,r=0.999 9;杂质D的线性范围为0.02~0.80 μg·mL-1,r=0.999 9。维生素D3杂质A~D的平均回收率(n=9)为93.2%~102.9%。对3批维生素D滴剂(软胶囊)样品进行检测,结果已知杂质及其他最大单个杂质含量均≤0.5%,杂质总含量≤1.0%。结论:本方法经系统方法学验证,适用于维生素D滴剂(软胶囊)中维生素D3有关物质的检测,且具有操作简便、快速、准确的优势,为脂溶性维生素D3制剂的质量控制提供了技术保证。
Objective: To establish a solid-phase extraction-high performance liquid chromatography(SPE-HPLC) method for the rapid detection of related substances of vitamin D3, trans vitamin D3 (impurity A), 7-dehydrocholesterol (impurity B), lumisterol 3 (impurity C), and isotachysterol 3 (impurity D), in vitamin D drops (soft capsules). Methods: The content, which was approximately equivalent to vitamin D3 1 548 IU, was taken in about 1.2 g, weighed accurately, put into a test tube, 4 mL of isooctane was added to it, and it was swirled and mixed well. Purification was performed using solid phase extraction columns, with n-hexane-ethyl acetate(85∶15) as the elution agent and anhydrous ethanol as the desorption agent. A Luna Silica(2) 100 Å silica gel column(150 mm×4.6 mm, 3 μm) was used, with n-hexane-n-pentanol (996.5∶3.5) as the mobile phase. The content of vitamin D3 impurities was calculated using the adding standard calibration factors principal component self-control method. Results: The separation degree between pre-vitamin D3 and vegetable oil was greater than 1.5. The linear range of vitamin D3 was 0.02-0.80 μg·mL-1, with r=0.999 5. The linear range of impurity A was 0.02 -0.80 μg·mL-1, with r=0.999 9. The linear range of impurity B was 0.02-0.80 μg·mL-1, with r=0.999 9. The linear range of impurity C was 0.02-0.80 μg·mL-1, with r=0.999 9. The linear range of impurity D was 0.02-0.80 μg·mL-1, with r=0.999 9. The average recovery rate of impurities A-D (n=9) was 93.2%-102.9%. The detection results of 3 batches of vitamin D drops (soft capsules) showed that the content of known impurities and other maximum single impurities were less than 0.5%, and the total content of impurities was less than 1.0%. Conclusion: The results indicate that the established method is suitable for the detection of vitamin D3 related substances in vitamin D drops (soft capsules). It has the advantages of being easy to operate, providing rapid and accurate results, and providing technical assurance for the quality control of fat-soluble vitamin D3 preparations.
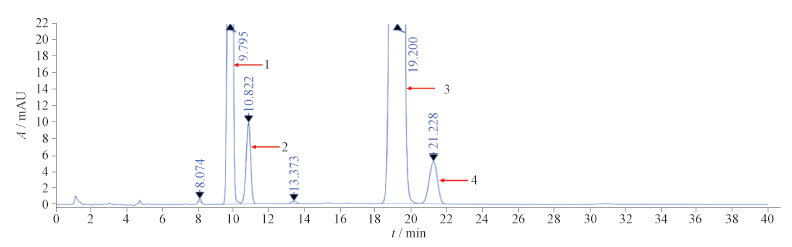
[1] 周璟明, 许福春, 陈秀明, 等. 高效液相色谱-串联三重四极杆质谱法测定维生素D/AD滴剂(软胶囊)中维生素D3的含量[J]. 药物分析杂志, 2022, 42(5): 780
ZHOU JM, XU FC, CHEN XM, et al. Determination of vitamin D3 in vitamin D/AD drops (soft capsules) by high performance liquid chromatography-tandem triple quadrupole mass spectrometry[J]. Chin J Pharm Anal, 2022, 42(5): 780
[2] 中华人民共和国药典 2020年版. 二部[S]. 2020: 1485
ChP 2020. Vol Ⅱ[S]. 2020: 1485
[3] USP 34- NF 29[S]. 2021: 2324
[4] EP 10.0[S]. 2013:2195
[5] 胡屹淼. 维生素D3相关杂质制备及其相对校正因子测定[D]. 杭州: 浙江大学, 2019
HU YM. Preparation and Relative Correction Factors Study of Vitamin D3 Impurities[D]. Hangzhou: Zhejiang University, 2019
[6] 王尚, 郑枫, 丁黎. RP-HPLC法测定维生素D3制剂中的有关物质[J]. 中国药科大学学报, 2012, 43(1): 55
WANG S, ZHENG F, DING L. Determination of related substances of vitamin D3 in its preparations by RP-HPLC [J]. J China Pharm Univ, 2012, 43(1): 55
[7] 张俊林, 邱科先. HPLC法测定维生素D3粉中有关物质[J]. 广东化工, 2021, 48(14): 229
ZHANG JL, QIU KX. Determination of related substances in vitamin D3 powder by HPLC [J]. Guangdong Chem Ind, 2021, 48(14): 229
[8] 李梦瀚, 丁雪梅, 梁建英. 反相高效液相色谱(RP-HPLC)系统分析维生素D3软胶囊(油性基质)[J]. 复旦学报: 医学版, 2019, 46(1): 23
LI MH, DING XM, LIANG JY. Reverse phase high-performance liquid chromatography(RP-HPLC) system for analysis of vitamin D3 soft capsules with soybean oil matrix[J]. Fudan Univ J Med Sci, 2019, 46(1): 23
[9] 洪雷洁, 石璐, 张亚雷, 等. 固相萃取-高效液相色谱法同时测定水体中的10种磺胺类抗生素[J]. 环境科学, 2012, 33(2): 652
HONG LJ, SHI L, ZHANG YL, et al. Simultaneous determination of 10 sulfonamide antibiotics in water by solid-phase extraction and high performance liquid chromatography[J]. Environ Sci, 2012, 33(2): 652
[10] 戚欣, 汪雪芳, 喻理, 等. 基于固相萃取的粮油产品前处理技术研究进展[J]. 中国油料作物学报, 2022, 44(4): 718
QI X, WANG XF, YU L, et al. Solid phase extraction based on sample preparation for grain, oilseed and their products[J]. Chin J Oil Crops Sci, 2022, 44(4): 718
[11] 康凯, 卢俊彪, 范国梁. 固相微萃取的发展近况[J]. 化学研究与应用, 2002, 14(4): 371
KANG K, LU JB, FAN GL. Advances of solid phase microextration[J]. Chem Res Appl, 2002, 14(4): 371
[12] 闫建康. HLB固相萃取-高效液相色谱法测定婴幼儿奶粉中对羟基苯甲酸酯类的研究[J]. 绿色科技, 2013, 15(12): 257
YAN JK. Determination of p-hydroxybenzoates in infant milk powder by using HLB solid-phase extraction-high performance liquid chromatography[J]. J Green Sci Technol, 2013, 15(12): 257
[13] 徐超, 周波, 王晓红. 固相萃取-高效液相色谱法测定大豆油中维生素K1含量[J]. 农业科技与装备, 2016(1): 53
XU C, ZHOU B, WANG XH. Determination of vitamin K1 content in soybean oil by solid phase extraction coupled with high performance liquid chromatography[J]. Agric Sci Technol Equip, 2016(1): 53
[14] 孙璐璐, 朱帅, 陈彦会, 等. 固相萃取-高效液相色谱法测定饼干中的维生素B12[J]. 食品安全质量检测学报, 2019, 10(15): 5081
SUN LL, ZHU S, CHEN YH, et al. Determination of vitamin B12 in biscuit by solid phase extraction-high performance liquid chromatography[J]. J Food Saf Qual, 2019, 10(15): 5081
[15] 黄旭, 刘家阳. 固相萃取-高效液相色谱法测定婴幼儿配方粉中维生素A和维生素E的含量[J]. 中国食品卫生杂志, 2018, 30(1): 38
HUANG X, LIU JY. Determination of vitamin A and vitamin E in infant formula powder by solid phase extraction and high performance liquid chromatography [J]. Chin J Food Hyg, 2018, 30(1): 38
[16] 刘铁成, 姜海涛, 杨蕾, 等. 一种维生素D3滴剂中维生素D3有关物质的检测方法:中国, N115308338A[P]. 2022-11-08
LIU TC, JIANG HT, YANG L, et al. A Detection Method for Vitamin D3 Related Substances in Vitamin D3 Drops: China, N115308338A[P]. 2022-11-08
[17] 中华人民共和国药典 2020年版. 四部[S]. 2020: 105
ChP 2020. Vol Ⅳ[S]. 2020: 105
[18] 肖亭, 王晨, 姚尚辰, 等. HPLC校正因子法在药物分析中的应用[J]. 药学学报, 2020, 55(12): 2854
XIAO T, WANG C, YAO SC, et al. Application of an HPLC correction factor method in pharmaceutical analysis [J]. Acta Pharm Sin, 2020, 55(12): 2854
[19] 刘莹, 刘丽兰, 王艳超, 等. 固相萃取-在线二维快速同时测定食品中维生素A、D和4种维生素E异构体的含量[J]. 中国标准化, 2019(18): 175
LIU Y, LIU LL, WANG YC, et al. Solid phase extraction-online two-dimensional rapid simultaneous determination of vitamin A, D, and four vitamin E isomers in food[J]. China Standard, 2019(18): 175
[20] 李少华, 申书昌, 戴建华. 正相固相萃取柱的制备及汉麻中CBD, CBN和THC的测定[J]. 齐齐哈尔大学学报(自然科学版), 2020, 36(6): 13
LI SH, SHEN SC, DAI JH. Preparation of normal phase solid phase extraction and determination of CBD, CBN and THC in hemp[J]. J Qiqihar Univ(Nat Sci Ed), 2020, 36(6): 13
[21] SONG H, WANG X, HOU S, et al. Economical irregular silica as an effective dispersive solid-phase extraction sorbent for the quantification of calcitriol in soft capsules[J]. J Pharm Biomed Anal, 2021, 5(203): 114227



