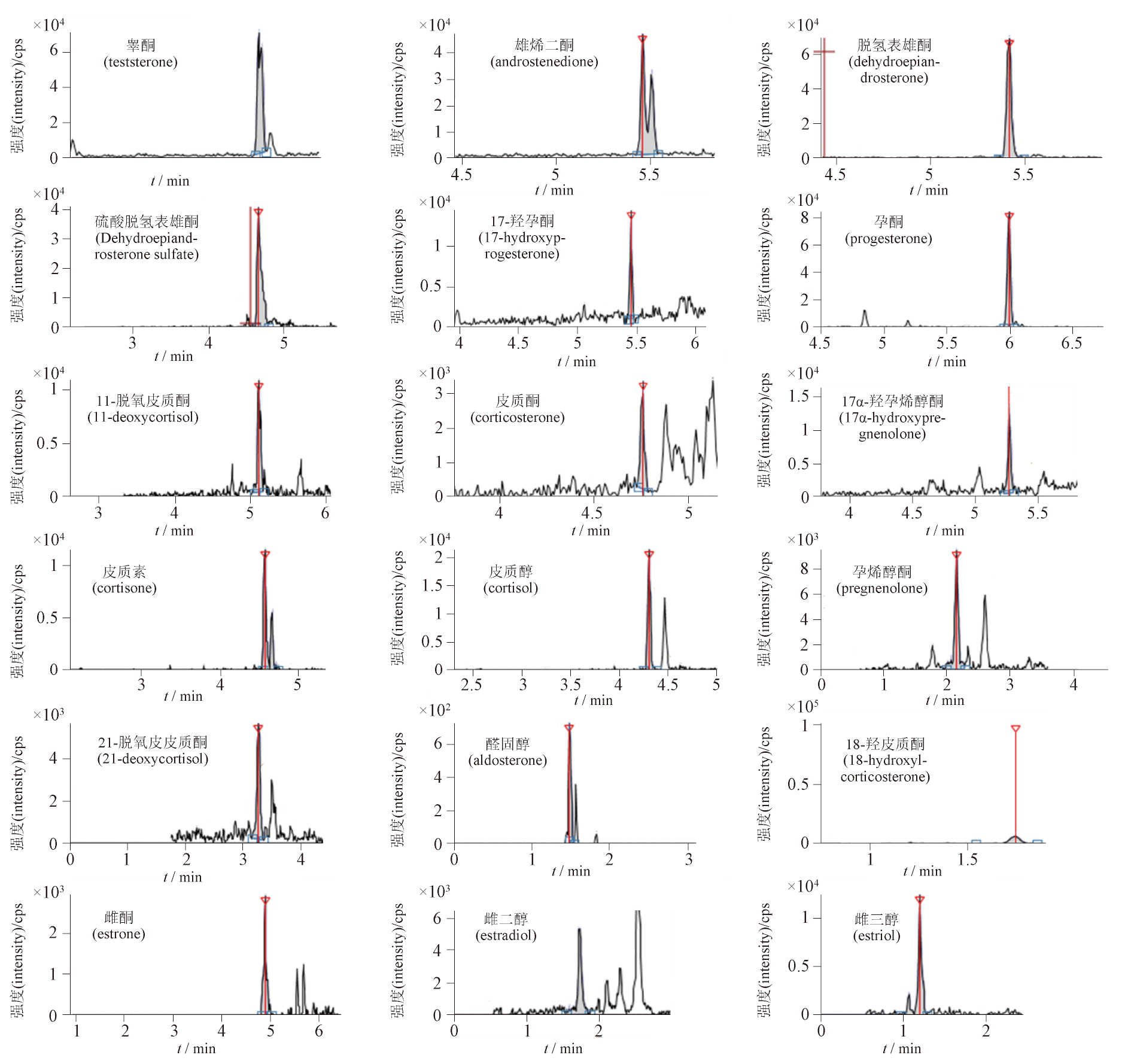
目的: 建立一种双衍生法-结合串联质谱技术同时检测人血清中18种类固醇激素的方法。方法: 血清样本采用羟胺和1,2-二甲基咪唑-5-磺酰氯对进行衍生处理,采用液相色谱-串联质谱法正离子选择离子监测模式(SRM)下检测,色谱柱为Kinetex®C8(100 mm×2.1 mm,2.6 μm),以0.1%甲酸水溶液为流动相A, 0.1%甲酸甲醇溶液为流动相B,梯度洗脱(0~0.5 min,35%B;0.5~5 min,35%B→100%B;5.0~5.1 min,100%B→35%B;5.1~7 min,35%B),柱温40 ℃,流速0.4 mL·min-1,进样量20 μL,采集时间为6 min。结果: 18种类固醇激素的线性相关系数均>0.99,回收率均在85%~115%,精密度RSD均<15%。用该方法成功地检测了156份健康人群受试者血清样本(男性75例,女性61例)并制定了参考区间。结论: 该方法可用非固相萃取法同时检测孕烯醇酮、17-羟孕烯醇酮、孕酮、17-羟孕酮、皮质酮、皮质醇、11-脱氧皮质醇、21-脱氧皮质醇、醛固酮、睾酮、雄烯二酮、脱氢表雄酮、硫酸脱氢表雄酮、皮质素、18-羟皮质酮、雌酮、雌二醇、雌三醇等18种类固醇激素。
Objective: To establish a dual derivatization method combined with tandem mass spectrometry for the simultaneous determination of 18 different steroid hormones in human serum. Methods: Serum samples were treated with hydroxylamine and 1,2-dimethylimidazole-5-sulfonyl chloride for derivatization, and the resulting compounds were analyzed by liquid chromatography-tandem mass spectrometry in positive ion selected reaction monitoring (SRM) mode. The Kinetex®C8 column (100 mm×2.1 mm, 2.6 μm) was used for the separation. Mobile phase A (0.1% acetic acid in water) and mobile phase B (0.1% acetic acid in methanol) with gradient elution(0-0.5 min,35%B;0.5-5 min, 35%B→100%B; 5.0-5.1 min,100%B→35%B; 5.1-7 min, 35%B)at the flow rate of 0.4 mL·min-1 were applied. Column temperature was set at 40 ℃. Injection volume was 20 μL and collection time was 6 min. Results: The linearity correlation coefficients for all 18 steroid hormones were greater than 0.99, the recovery rates ranged from 85% to 115%, and the precision RSD was less than 15%. This method was successfully applied to the analysis of serum samples from 156 healthy subjects (75 males and 61 females), and reference intervals were established. Conclusion: This method can be used to simultaneously determine 18 types of steroid hormones, such as pregnenolone, 17-hydroxypregnenolone, progesterone, 17-hydroxyprogesterone, corticosterone, cortisol, 11-deoxycorticosterone, 21-deoxycorticosterone, aldosterone, testosterone, androstanedione, dehydroepiandrosterone, sulfated dehydroepiandrosterone, adrenocorticotropin, 18-hydroxicorticotropin, estrone, estradiol, and estriol using non-solid-phase extraction.
[1] WEHLING M. Nongenomic actions of steroid hormones[J]. Trends Endocrinol Metabol, 1994, 5(8): 347
[2] SHORE LS,SHEMESH M. Naturally produced steroid hormones and their release into the environment[J]. Pure Appl Chem, 2003, 75(11-12): 1859
[3] ELIASSEN AH, SPIEGELMAN D, XU X, et al. Urinary estrogens and estrogen metabolites and subsequent risk of breast cancer among premenopausal women[J]. Cancer Res, 2012, 72(3): 696
[4] NESHER M, SHPOLANSKY U, ROSEN H, et al. The digitalis-like steroid hormones: new mechanisms of action and biological significance.[J]. Life Sci, 2007,80(23):2093
[5] ZOGRAFOS GN, PERYSINAKIS I, VASSILATOU E. Subclinical Cushing's syndrome: current concepts and trends.[J]. Hormones, 2014, 13(3):323
[6] REINCKE M. Subclinical Cushing's syndrome[J]. Endocrinol Metabol Clin North Am, 2000, 29(1): 43
[7] FANELLI F, BELLUOMO I, DI LALLO VD, et al. Serum steroid profiling by isotopic dilution-liquid chromatography-mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults[J]. Steroids, 2011, 76(3): 244
[8] HANDELSMAN DJ, WARTOFSKY L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism[J]. J Clin Endocrinol Metabol, 2013, 98(10): 3971
[9] PAGOTTO U. Mass spectrometry in the measurement of hormones[J]. Endocrine, 2011.
[10] SCHIFFER L, BARNARD L, BARANOWSKI ES,et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review[J]. J Steroid Biochem Molecul Biol, 2019,194:105439
[11] HIGASHI T, OGAWA S. Chemical derivatization for enhancing sensitivity during LC/ESI-MS/MS quantification of steroids in biological samples: a review[J]. J Steroid Biochem Molecul Biol, 2016, 162: 57
[12] GAUDL A, JÜRGEN KRATZSCH, CEGLAREK U. Advancement in steroid hormone analysis by LC-MS/MS in clinical routine diagnostics-A three year recap from serum cortisol to dried blood 17α-hydroxyprogesterone[J]. J Steroid Biochem Molecul Biol, 2019, 192:105389
[13] MOLNÁR S, KULCSÁR G, PERJÉSI P. Determination of steroid hormones in water samples by liquid chromatography electrospray ionization mass spectrometry using parallel reaction monitoring[J]. Microchem J, 2022,175: 107105
[14] YUAN M, BREITKOPF SB, YANG X, et al. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue [J]. Nat Protocols, 2012, 7(5):872
[15] NALDI AC, FAYAD PB, PRÉVOST M, et al. Analysis of steroid hormones and their conjugated forms in water and urine by on-line solid-phase extraction coupled to liquid chromatography tandem mass spectrometry[J]. Chem Cent J, 2016, 10(1): 1
[16] FERRERA ZS, SANTANA CM, JOSÉ JSR. Steroid Hormones in Biological and Environmental Samples: Extraction and Determination Techniques[M]. Steroids: Biosynthesis, Functions and Health Implications, 2012:83
[17] QIN Q, FENG D, HU C, et al. Parallel derivatization strategy coupled with liquid chromatography-mass spectrometry for broad coverage of steroid hormones[J]. J Chromatogr A, 2020, 1614: 460709
[18] HÄKKINEN M R, MURTOLA T, VOUTILAINEN R, et al. Simultaneous analysis by LC-MS/MS of 22 ketosteroids with hydroxylamine derivatization and underivatized estradiol from human plasma, serum and prostate tissue[J]. J Pharm Biomed Anal, 2019, 164: 642
[19] KIOUSI P, FRAGKAKI AG, KIOUKIA-FOUGIA N, et al. Liquid chromatography-mass spectrometry behavior of Girard's reagent T derivatives of oxosteroid intact phase Ⅱ metabolites for doping control purposes[J]. Drug Test Anal, 2021, 13(11-12): 1822
[20] ZHAO XE, YAN P, WANG R, et al. Sensitive determination of cholesterol and its metabolic steroid hormones by UHPLC-MS/MS via derivatization coupled with dual ultrasonic-assisted dispersive liquid-liquid microextraction[J]. Rapid Commun Mass Spectr, 2016, 30: 147
[21] PETUCCI C, LLOYD T, HARRIS HA, et al. Trace LC/MS/MS quantitation of 17β-estradiol as a biomarker for selective estrogen receptor modulator activity in the rat brain[J]. J Mass Spectr, 2010, 45(1): 65
[22] KESKI-RAHKONEN P, DESAI R, JIMENEZ M, et al. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent[J]. Anal Chem, 2015, 87(14): 7180
[23] SHOU WZ, JIANG X, NAIDONG W. Development and validation of a high-sensitivity liquid chromatography/tandem mass spectrometry (LC/MS/MS) method with chemical derivatization for the determination of ethinyl estradiol in human plasma[J]. Biomed Chromatogr, 2010, 18(7):414
[24] WS/T 402-2012 临床实验室检验项目参考区间的制定[S]. 2012
WS/T 402-2012 Define and Determine the Reference Intervals in Clinical Laboratory [S]. 2012
[25] TAYLOR AE, KEEVIL B, HUHTANIEMI IT. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow[J]. Eur J Endocrinol, 2015, 173(2): D1
[26] DAI W, HUANG Q, YIN P, et al. Comprehensive and highly sensitive urinary steroid hormone profiling method based on stable isotope-labeling liquid chromatography mass spectrometry[J]. Anal Chem, 2012, 84(23):10245



